library(dartRverse)
library(dartR.sexlinked)1 Importing and Handling Genomic Data
Session Presenters

Required packages
Introduction
SNPs, or single nucleotide polymorphisms, are single base pair mutations at a nuclear locus (Figure 2). That nuclear locus is represented in the dataset by two sequence tags which, at a heterozygous locus, take on two allelic states, one referred to as the reference state, the other as the alternate or SNP state.
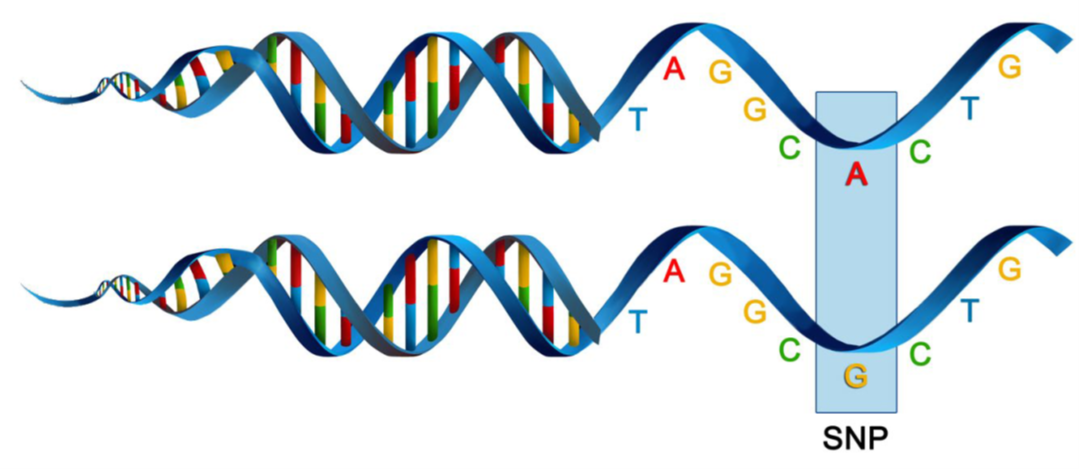
Because it is extremely rare for a mutation to occur twice at the same site in the genome (perhaps with the exception of Eucalypts), the SNP data are considered to be effectively biallelic. Sites with more than two states that occur rarely are typically eliminated in the quality control steps as they are bundled with multiallelic sites arising from multiple copy sequences (e.g. as would arise from gene duplications) removed during preliminary filtering.
The data can be represented as SNP bases (A, T, C or G), with two states for each individual at each locus in a diploid organisms. Alternatively, because the data are biallelic, it is computationally convenient to code the data as 0 for homozyogotes for one allele, 1 for heterozygotes, and 2 for homozygotes of the other allele. The reference allele is arbitrarily taken to be the most common allele, so 0 is the score for homozygous reference, and 2 is the score for homozygous alternate or SNP state. NA indicates that the SNP could not be scored.
SNP data when using dartR are held in a genlight object that is defined in R package adegenet (Jombart, 2008; Jombart and Ahmed, 2011). The locus metadata included in the genlight object are those provided as part of your Diversity Arrays Technology report. These metadata are obtained from the Diversity Arrays Technology csv file when it is read in to the genlight object. The locus metadata are held in an R data.frame that is associated with the SNP data as part of the genlight object.
Here is an example of the data structure of a genlight object
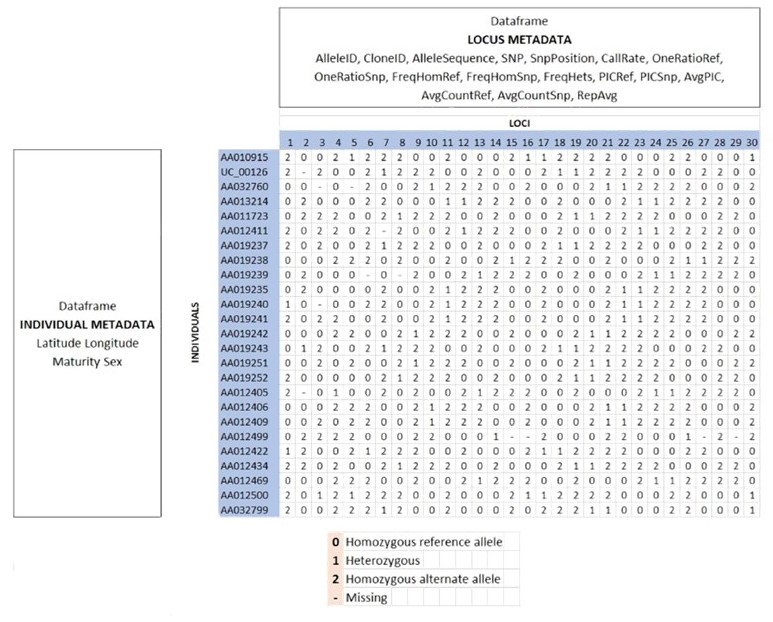
For further details go check out the Data Structure tutorial.
dartR Fundamentals
To help us get our head around data interrogation, subsetting, and basic filtering we are going to work through a worked example using a test dataset.
# dartR comes with a built in test dataset called testset.gl.
# We first examine the contents of testset.gl
testset.gl![]() This is referring to the
This is referring to the testset.gl data set you have interrogated above.
How many individuals have been genotyped?
How many SNP loci have been scored?
What is the percentage of missing data?
# Next let us copy the contents of testset.gl to another genlight object called gl.
gl <- testset.gl
# Use adegenet accessors to interrogate the genlight object further
nInd(gl)
nLoc(gl)
nPop(gl)
popNames(gl)
indNames(gl)
locNames(gl)Now you have a better appreciation of the contents of genlight object gl. pop(gl) is a vector of population names against each individual. Note that it is distinguished from popNames(gl) which just lists the unique names.
# So if you want to tablulate the number of individuals in each population, use
table(pop(gl))
EmmacBrisWive EmmacBurdMist EmmacBurnBara EmmacClarJack
10 10 11 5
EmmacClarYate EmmacCoopAvin EmmacCoopCully EmmacCoopEulb
5 10 10 10
EmmacFitzAllig EmmacJohnWari EmmacMaclGeor EmmacMaryBoru
10 10 11 6
EmmacMaryPetr EmmacMDBBowm EmmacMDBCond EmmacMDBCudg
4 10 10 10
EmmacMDBForb EmmacMDBGwyd EmmacMDBMaci EmmacMDBMurrMung
11 9 10 10
EmmacMDBSanf EmmacNormJack EmmacNormLeic EmmacNormSalt
10 6 1 1
EmmacRichCasi EmmacRoss EmmacRussEube EmmacTweeUki
10 10 10 10
EmsubRopeMata EmvicVictJasp
5 5 We have learned that typing the name of the genlight object gives its attributes. How do we examine the genotypes in a genlight object. This is done by converting it to a matrix.
as.matrix(gl)[1:7,1:4] 100049687-12-C/T 100049698-16-G/A 100049728-23-A/G 100049805-56-T/A
AA010915 2 NA 0 0
UC_00126 2 NA 0 0
AA032760 NA NA 0 0
AA013214 2 NA 0 0
AA011723 2 NA 0 0
AA012411 2 NA 0 0
AA019237 2 NA 0 0What do you see? Is it what you expected for the coding of the SNP scores?
Reporting
Lets start over with a focus on reporting.
# Copy the original testset.gl
gl <- testset.gl
# Set the global verbosity to 3 – this will result in some detailed comments
# as you run each # script
gl.set.verbosity(3)
# Now lets use a report function to report the call rate for genlight object gl
gl.report.callrate(gl)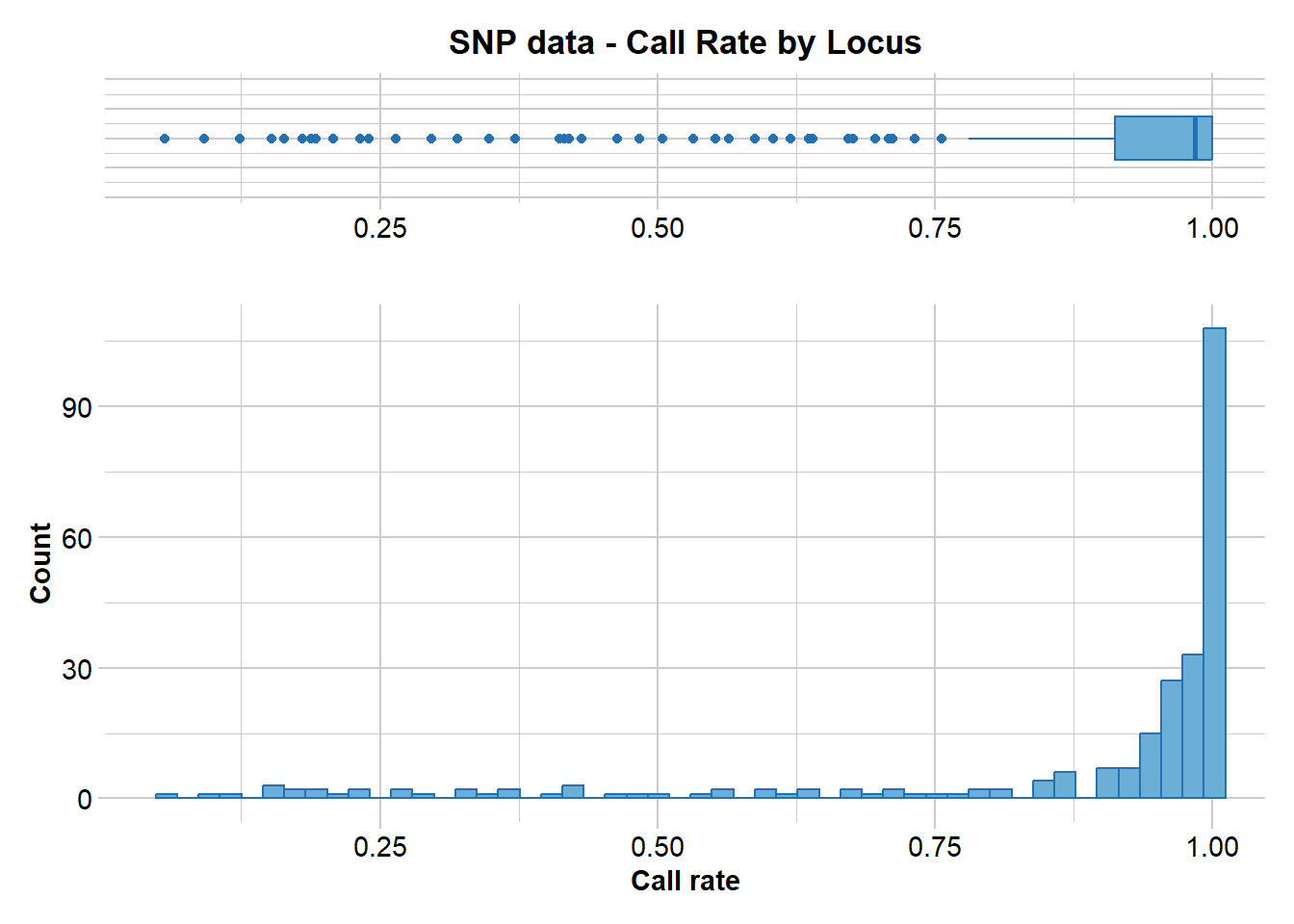
Note that the call rate is reported against locus. This is the default. Both a tabulated result and a graph is produced. You can use both to determine a suitable threshold for filtering on callrate.
What threshold do you think might be appropriate?
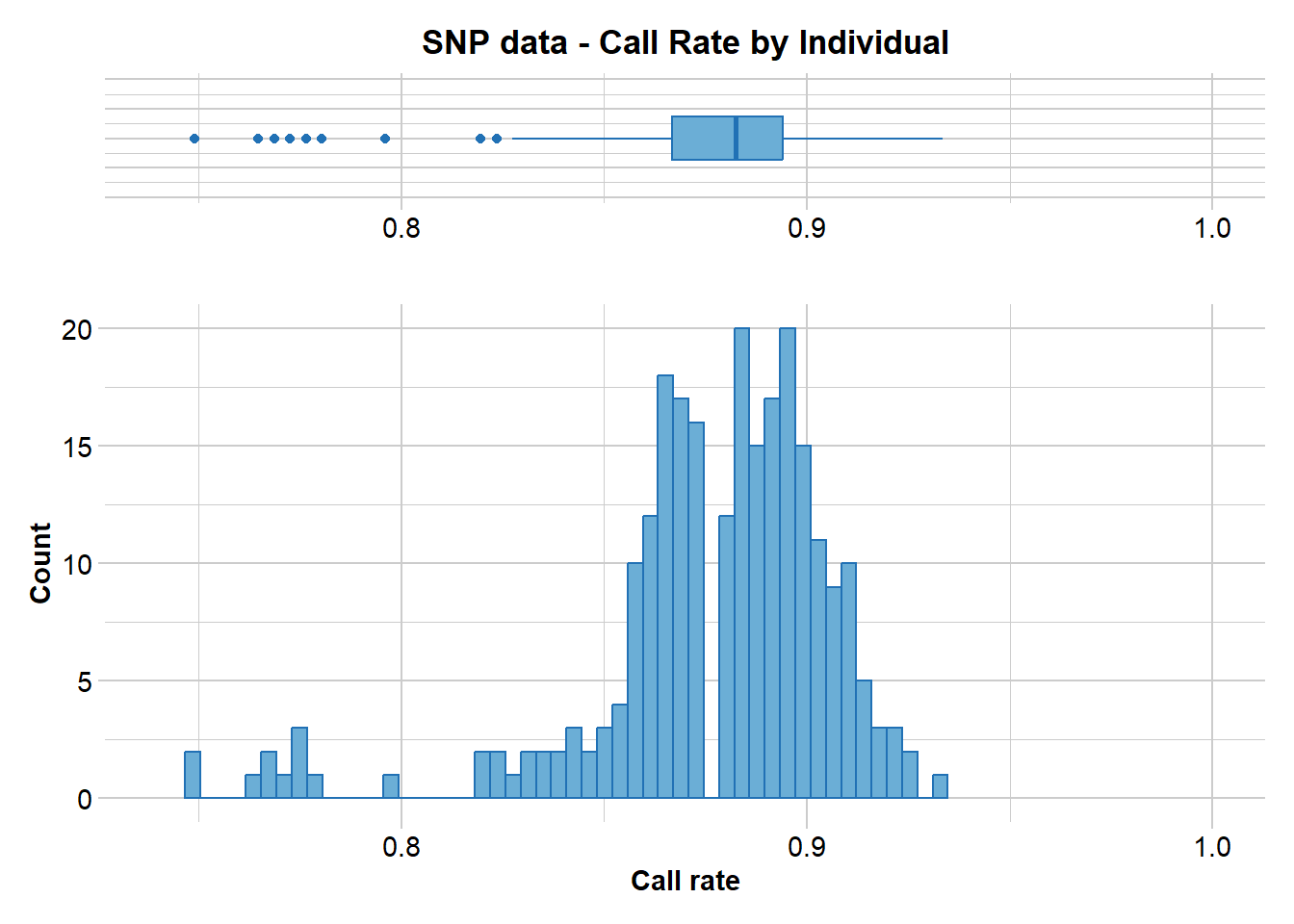
Now let’s report the call rate for genlight object gl by individual.
gl.report.callrate(gl,method="ind")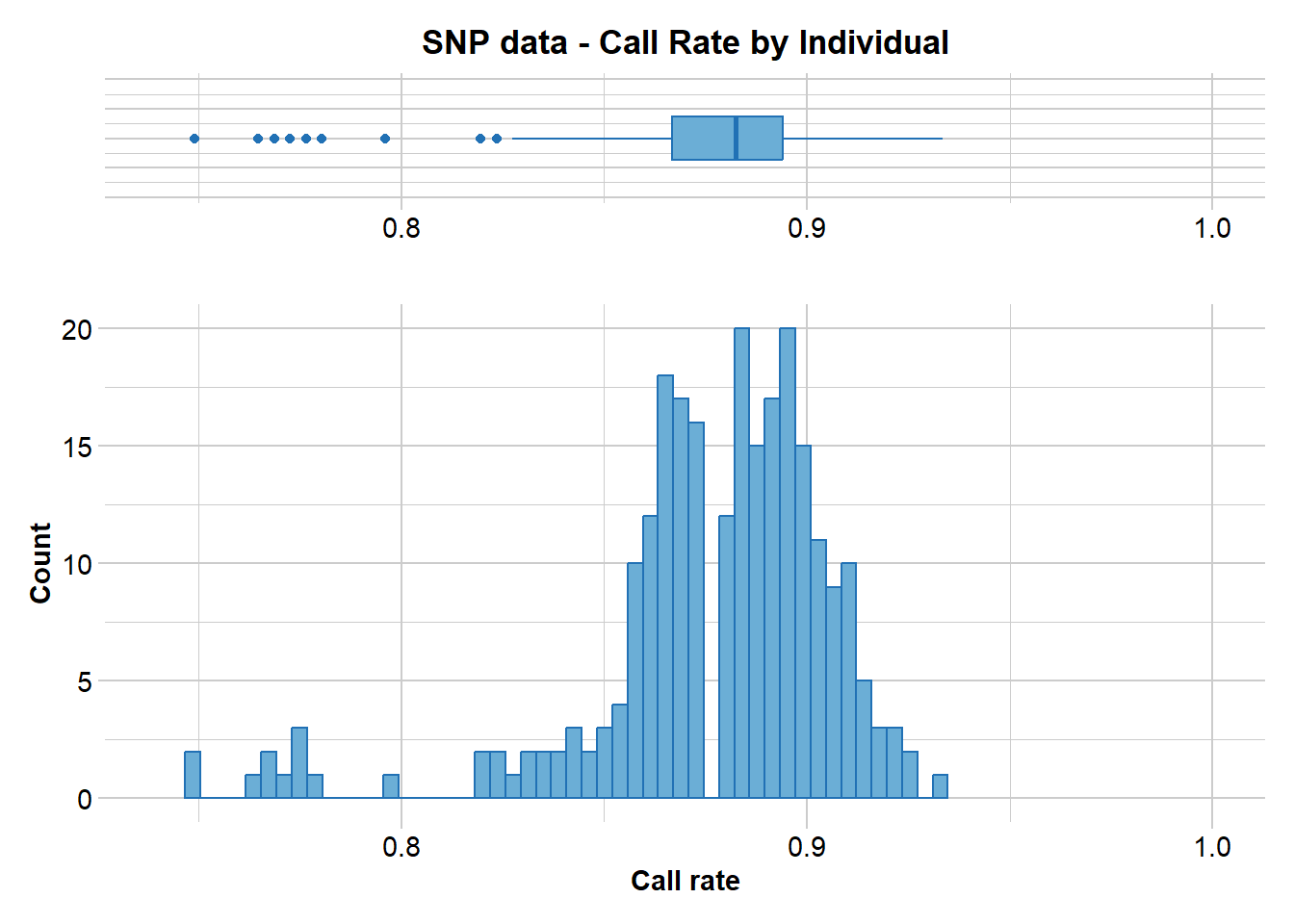
Can you see the difference. Again, both a tabulated result and a graph is produced. You can use both to determine a suitable threshold for filtering on callrate. What threshold do you think might be appropriate?
What about reproducibility?
gl.report.reproducibility(gl)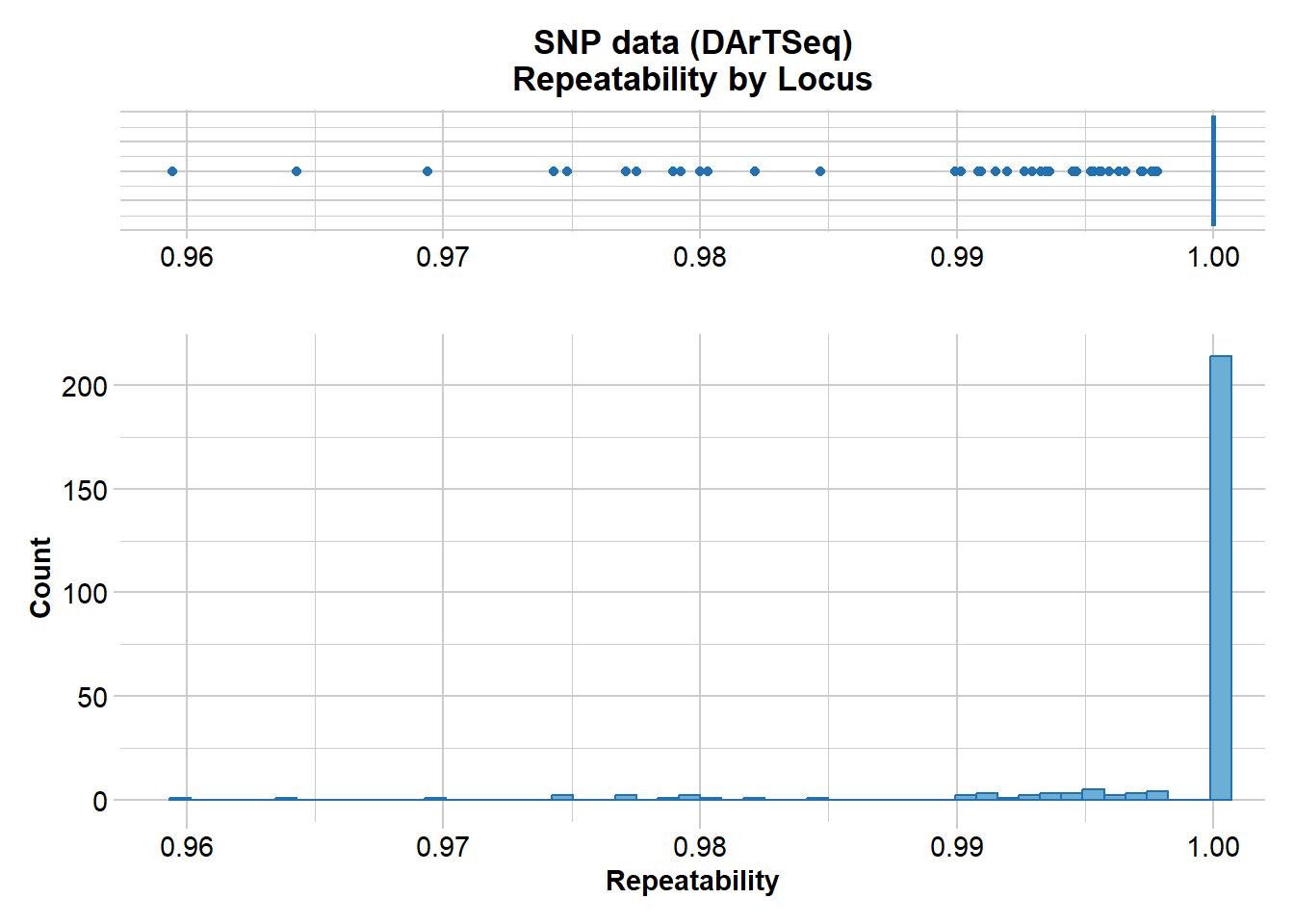
Recall that DArT runs a series of technical replicates as part of its routine workflow. This enables an assessment of the quality of data associated with each locus. What threshold do you think might be appropriate for a filter of reproducability?
Filtering
Now let’s try some filtering. Return to call rate.
# First look at a report to decide a threshold
gl.report.callrate(gl,method="ind")Starting gl.report.callrate
Processing genlight object with SNP data
Reporting Call Rate by Individual
No. of loci = 255
No. of individuals = 250
Minimum : 0.7490196
1st quartile : 0.8666667
Median : 0.8823529
Mean : 0.8765804
3r quartile : 0.8941176
Maximum : 0.9333333
Missing Rate Overall: 0.1234
Listing 30 populations and their average CallRates
Monitor again after filtering
Population CallRate N
1 EmmacBrisWive 0.8839 10
2 EmmacBurdMist 0.8808 10
3 EmmacBurnBara 0.8859 11
4 EmmacClarJack 0.8596 5
5 EmmacClarYate 0.8769 5
6 EmmacCoopAvin 0.7682 10
7 EmmacCoopCully 0.9122 10
8 EmmacCoopEulb 0.8702 10
9 EmmacFitzAllig 0.8973 10
10 EmmacJohnWari 0.8929 10
11 EmmacMaclGeor 0.8806 11
12 EmmacMaryBoru 0.8843 6
13 EmmacMaryPetr 0.8892 4
14 EmmacMDBBowm 0.8824 10
15 EmmacMDBCond 0.8855 10
16 EmmacMDBCudg 0.8878 10
17 EmmacMDBForb 0.8766 11
18 EmmacMDBGwyd 0.9050 9
19 EmmacMDBMaci 0.8773 10
20 EmmacMDBMurrMung 0.8890 10
21 EmmacMDBSanf 0.8914 10
22 EmmacNormJack 0.8725 6
23 EmmacNormLeic 0.8863 1
24 EmmacNormSalt 0.8706 1
25 EmmacRichCasi 0.8757 10
26 EmmacRoss 0.8706 10
27 EmmacRussEube 0.8612 10
28 EmmacTweeUki 0.8773 10
29 EmsubRopeMata 0.8345 5
30 EmvicVictJasp 0.8361 5
Listing 20 individuals with the lowest CallRates
Use this list to see which individuals will be lost on filtering by individual
Set ind.to.list parameter to see more individuals
Individual Population CallRate
1 AA063722 EmmacCoopAvin 0.7490196
2 AA063726 EmmacCoopAvin 0.7490196
3 AA063732 EmmacCoopAvin 0.7647059
4 AA063720 EmmacCoopAvin 0.7686275
5 AA063712 EmmacCoopAvin 0.7686275
6 AA063708 EmmacCoopAvin 0.7725490
7 AA063718 EmmacCoopAvin 0.7764706
8 AA063710 EmmacCoopAvin 0.7764706
9 AA063714 EmmacCoopAvin 0.7764706
10 AA063716 EmmacCoopAvin 0.7803922
11 AA032760 EmmacMDBMaci 0.7960784
12 UC_00210 EmsubRopeMata 0.8196078
13 UC_00259 EmvicVictJasp 0.8196078
14 AA018494 EmmacRichCasi 0.8235294
15 UC_00206 EmsubRopeMata 0.8235294
16 AA019164 EmmacRussEube 0.8274510
17 UC_00209 EmsubRopeMata 0.8313725
18 UC_00254 EmvicVictJasp 0.8313725
19 AA019159 EmmacRussEube 0.8352941
20 UC_00126c EmvicVictJasp 0.8352941
)
Completed: gl.report.callrate # Then filter using that threshold
gl <- gl.filter.callrate(gl, method="ind", threshold=0.80)Starting gl.filter.callrate
Processing genlight object with SNP data
Warning: Data may include monomorphic loci in call rate
calculations for filtering
Recalculating Call Rate
Removing individuals based on Call Rate, threshold = 0.8
Individuals deleted (CallRate <= 0.8 ):
AA032760[EmmacMDBMaci], AA063718[EmmacCoopAvin], AA063720[EmmacCoopAvin], AA063722[EmmacCoopAvin], AA063726[EmmacCoopAvin], AA063732[EmmacCoopAvin], AA063708[EmmacCoopAvin], AA063710[EmmacCoopAvin], AA063712[EmmacCoopAvin], AA063714[EmmacCoopAvin], AA063716[EmmacCoopAvin],
Summary of filtered dataset
Call Rate for individuals > 0.8
Original No. of loci : 255
Original No. of individuals: 250
No. of loci retained: 255
No. of individuals retained: 239
No. of populations: 29 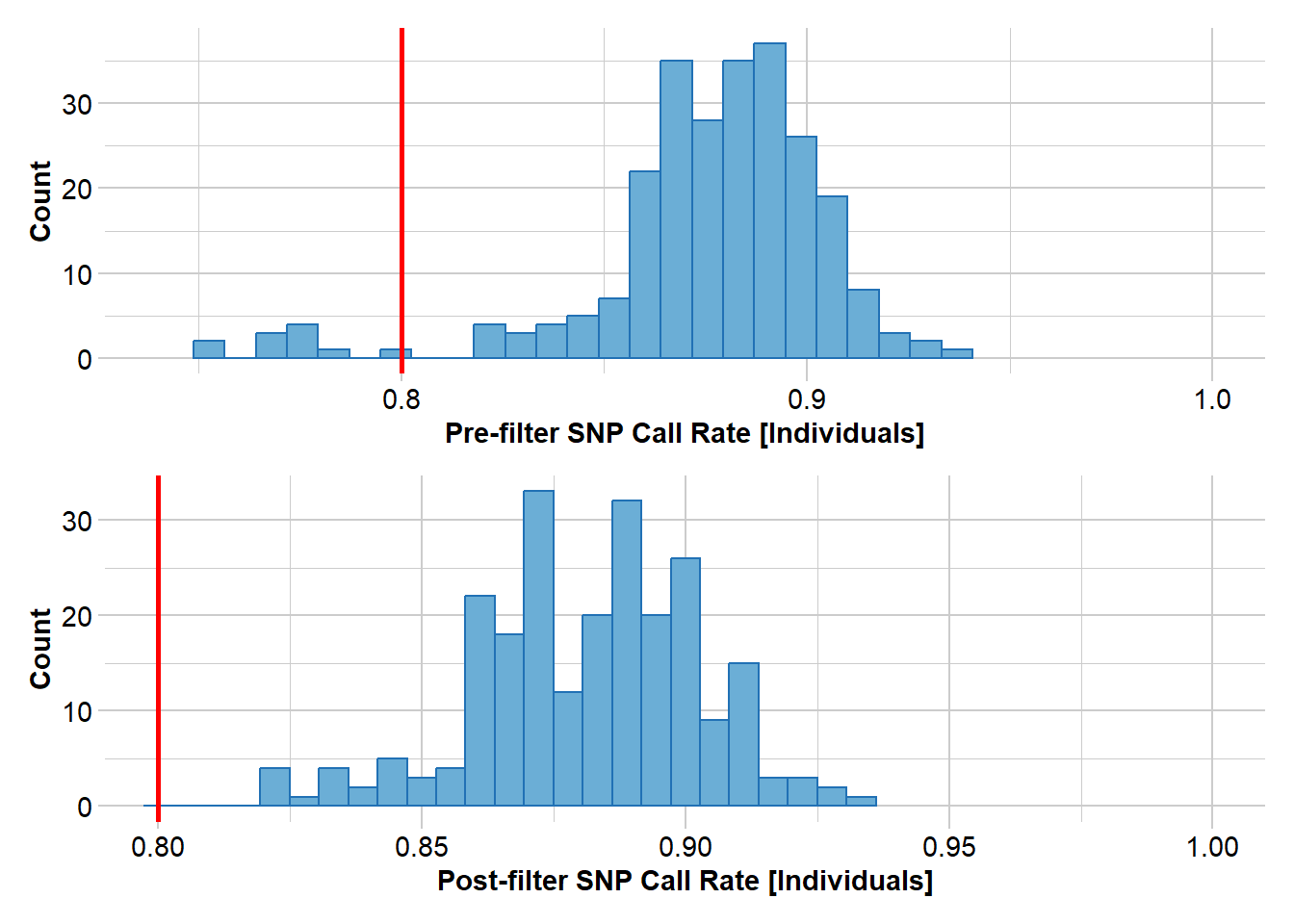
Note: Locus metrics not recalculated
Note: Resultant monomorphic loci not deleted
Completed: gl.filter.callrate # Use a smear plot for a visual assessment of the effectiveness of filtering
gl <- testset.gl
smear_pre <- gl.smearplot(gl) Processing genlight object with SNP data
Starting gl.smearplot 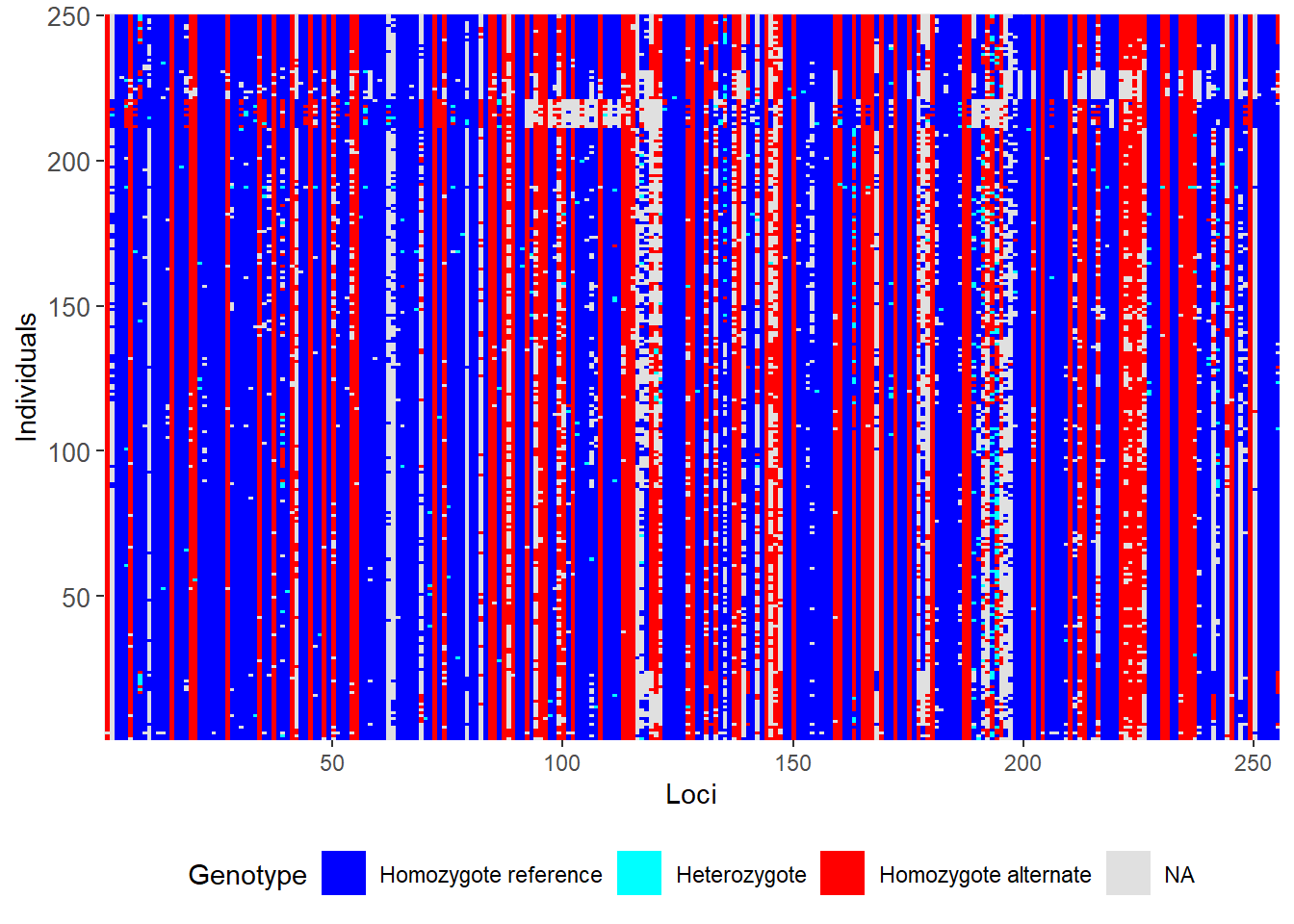
Completed: gl.smearplot Note the whitespace, which indicates missing data.
# Filter on callrate by locus, then on individual
gl <- gl.filter.callrate(gl,verbose=0)
gl <- gl.filter.callrate(gl, method= "ind", threshold=0.80, verbose=0)
# Examine the smearplot again
smear_post <- gl.smearplot(gl)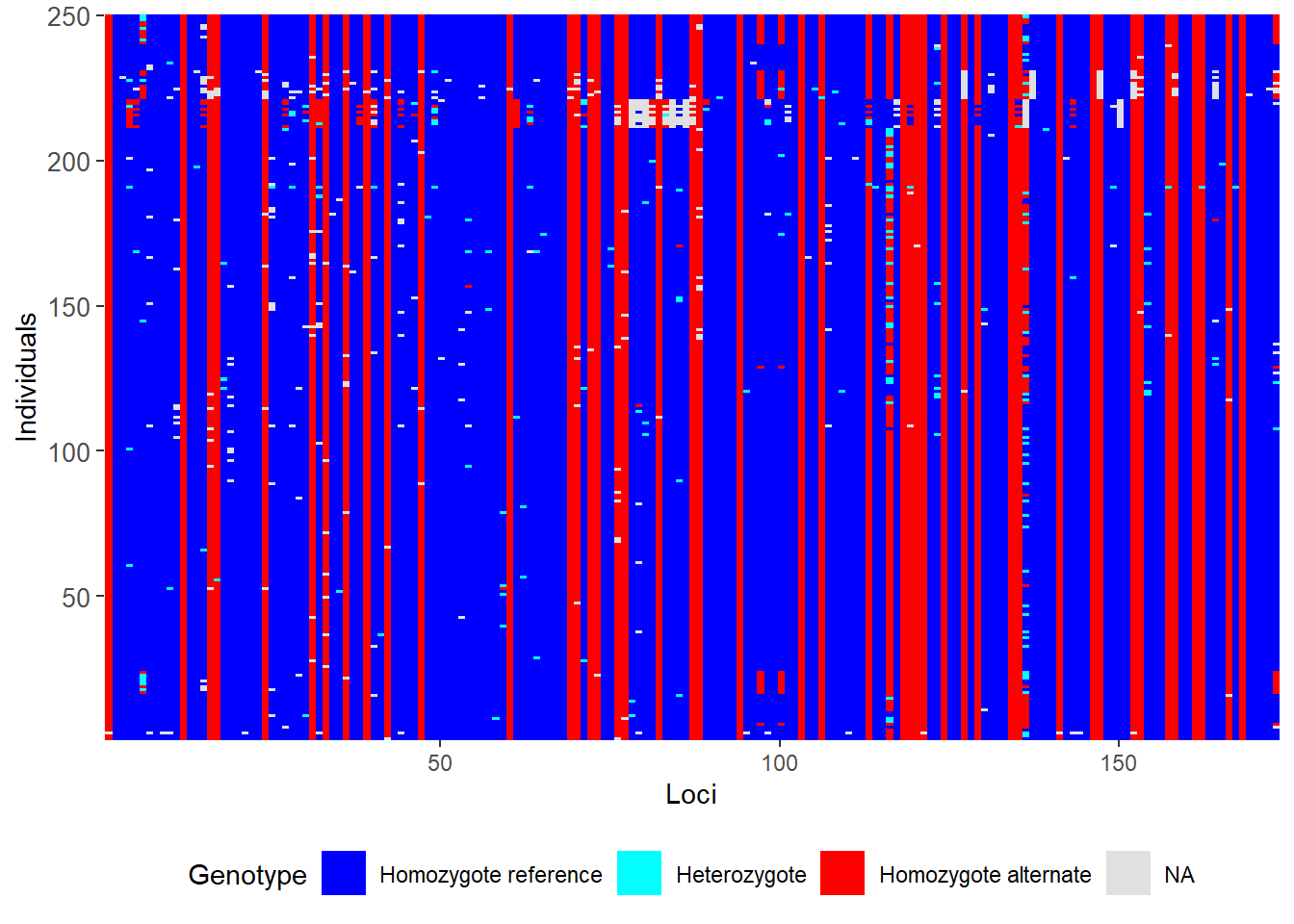
What has happened. What do you conclude?
Filtering out sex-linked markers
For this exercise we will use the data of the Leadbeater’s possum (LBP). This data is included in the package dartR.sexlinked

Load data
LBP # Explore the dataset
LBP@n.loc # Number of SNPs
length(LBP@ind.names) # Number of individuals![]()
How many SNPs and individuals does the LBP genlight object have?
Run gl.report.sexlinked
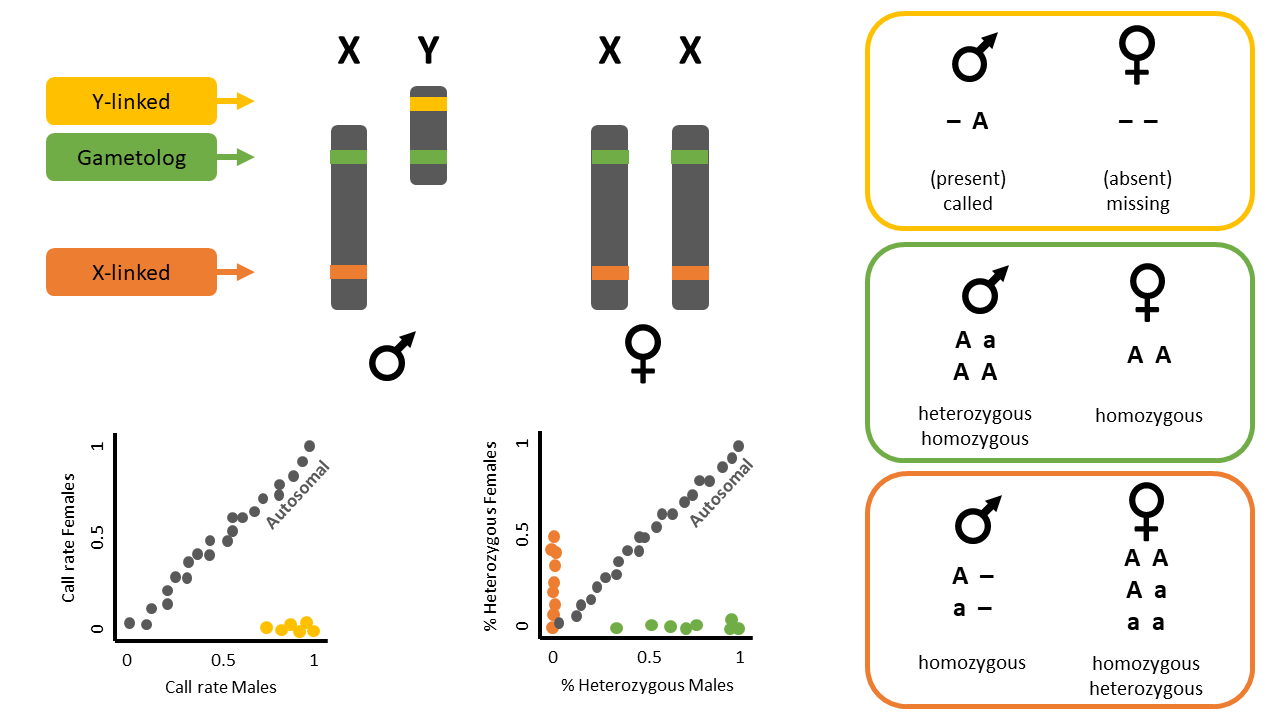
This function identifies sex-linked and autosomal loci present in a SNP dataset (i.e., genlight object) using individuals with known sex. It identifies five types of loci: w-linked or y-linked, sex-biased, z-linked or x-linked, gametologous and autosomal.
The genlight object must contain in gl@other$ind.metrics a column named id, and a column named sex in which individuals with known-sex are assigned M for male, or F for female. The function ignores individuals that are assigned anything else or nothing at all (unknown-sex).
![]() Check that
Check that ind.metrics has the necessary columns:
head(LBP@other$ind.metrics)| id | sex | pop | Year.collected | service | plate_location | |
|---|---|---|---|---|---|---|
| Y2 | Y2 | F | Yellingbo | 1997 | DLpos17-2786 | 1-A1 |
| Y16 | Y16 | M | Yellingbo | 2001 | DLpos17-2786 | 1-A10 |
| Y17 | Y17 | F | Yellingbo | 1997 | DLpos17-2786 | 1-A11 |
| Y18 | Y18 | F | Yellingbo | 1999 | DLpos17-2786 | 1-A12 |
| Y3 | Y3 | F | Yellingbo | 1997 | DLpos17-2786 | 1-A2 |
| Y4 | Y4 | M | Yellingbo | 1997 | DLpos17-2786 | 1-A3 |
![]() Check the manual of function
Check the manual of function gl.report.sexlinked to investigate its other requirements:
help(gl.report.sexlinked)Run the function to identify sex-linked loci in the LBP genlight object:
out <- gl.report.sexlinked(LBP, system = "xy")Starting gl.report.sexlinked
Processing genlight object with SNP dataDetected 162 females and 211 males.Starting phase 1. May take a while...Building call rate plot.Done building call rate plot.Starting phase 2. May take a while...Building heterozygosity plot.Done building heterozygosity plot.**FINISHED** Total of analyzed loci: 1000.
Found 77 sex-linked loci:
1 Y-linked loci (yellow)
9 sex-biased loci (blue)
66 X-linked loci (orange)
1 gametologs (green).
And 923 autosomal loci (grey).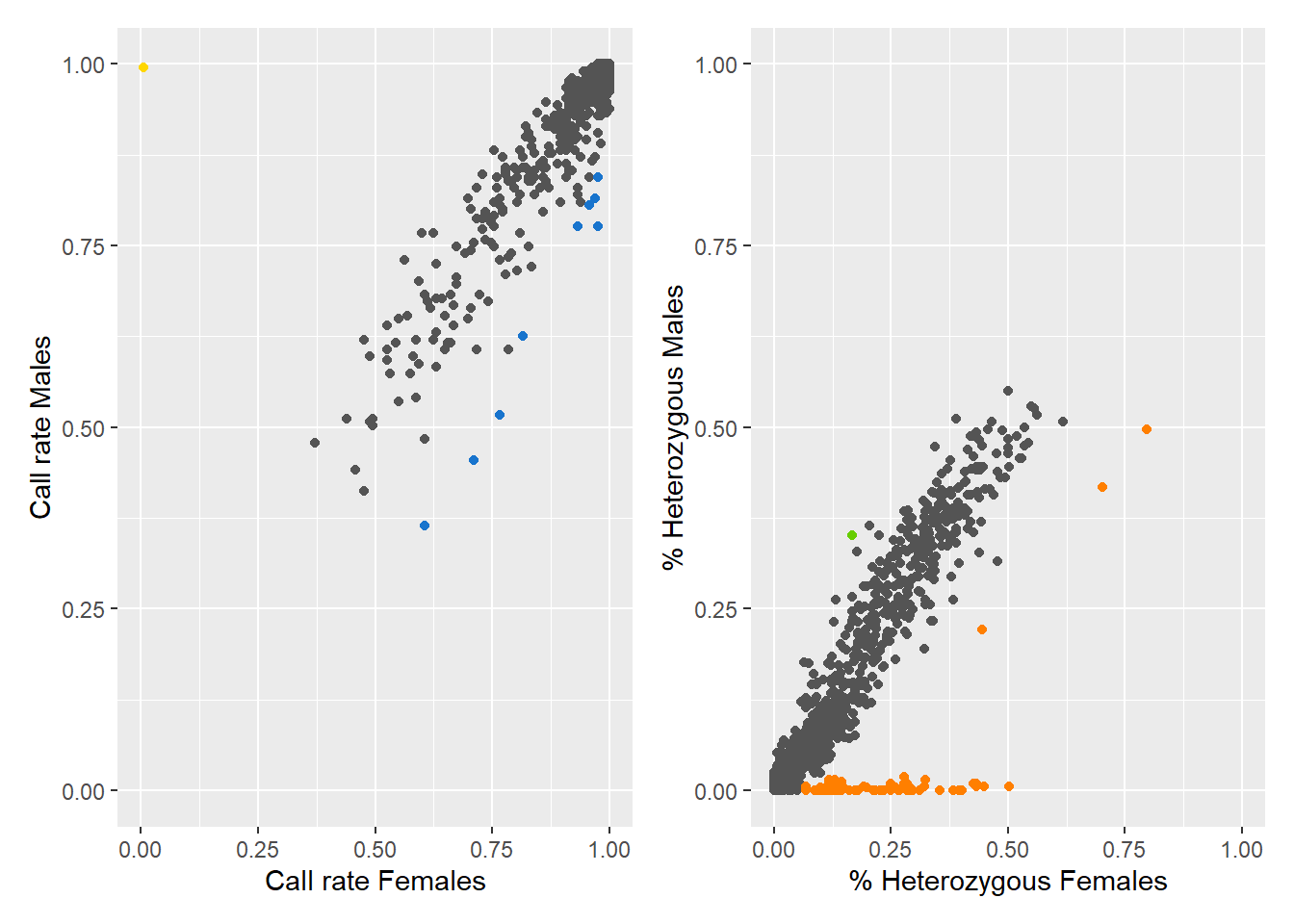
Completed: gl.report.sexlinked ![]()
Question: Why are we using “xy”?
Question: How many males and females does the dataset contain?
Question: How many sex-linked loci were found?
The output consists of two plots, plus a table. Examine the plots.
Question: What do the colours mean in the plots? Look at the figure below for a hint.
Output dataframe
Now check the output table
out| index | count.F.miss | count.M.miss | count.F.scored | count.M.scored | ratio | p.value | p.adjusted | scoringRate.F | scoringRate.M | y.linked | sex.biased | count.F.het | count.M.het | count.F.hom | count.M.hom | stat | stat.p.value | stat.p.adjusted | heterozygosity.F | heterozygosity.M | x.linked | gametolog | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 28681424-34-G/T | 1 | 0 | 1 | 162 | 210 | 1.2953770 | 1.0000000 | 1 | 1.0000000 | 0.9952607 | FALSE | FALSE | 1 | 0 | 161 | 210 | 1.303397 | 1.0000000 | 1.0000000 | 0.0061728 | 0.0000000 | FALSE | FALSE |
| 28678947-56-C/T | 2 | 12 | 8 | 150 | 203 | 2.0261283 | 0.1638428 | 1 | 0.9259259 | 0.9620853 | FALSE | FALSE | 9 | 7 | 141 | 196 | 1.784196 | 0.3044519 | 0.9598961 | 0.0600000 | 0.0344828 | FALSE | FALSE |
| 28680567-32-T/G | 3 | 12 | 12 | 150 | 199 | 1.3256351 | 0.5289429 | 1 | 0.9259259 | 0.9431280 | FALSE | FALSE | 9 | 11 | 141 | 188 | 1.090635 | 1.0000000 | 1.0000000 | 0.0600000 | 0.0552764 | FALSE | FALSE |
| 28688313-7-C/G | 4 | 0 | 0 | 162 | 211 | 1.3015303 | 1.0000000 | 1 | 1.0000000 | 1.0000000 | FALSE | FALSE | 6 | 0 | 156 | 211 | 8.076192 | 0.0459068 | 0.3917911 | 0.0370370 | 0.0000000 | FALSE | FALSE |
| 28681679-51-C/T | 5 | 22 | 30 | 140 | 181 | 0.9482168 | 0.8814171 | 1 | 0.8641975 | 0.8578199 | FALSE | FALSE | 1 | 1 | 139 | 180 | 1.293900 | 1.0000000 | 1.0000000 | 0.0071429 | 0.0055249 | FALSE | FALSE |
| 28681994-14-G/A | 6 | 0 | 1 | 162 | 210 | 1.2953770 | 1.0000000 | 1 | 1.0000000 | 0.9952607 | FALSE | FALSE | 18 | 19 | 144 | 191 | 1.255791 | 0.6007790 | 1.0000000 | 0.1111111 | 0.0904762 | FALSE | FALSE |
| 28687273-35-G/A | 7 | 2 | 2 | 160 | 209 | 1.3052926 | 1.0000000 | 1 | 0.9876543 | 0.9905213 | FALSE | FALSE | 64 | 81 | 96 | 128 | 1.053325 | 0.8302485 | 1.0000000 | 0.4000000 | 0.3875598 | FALSE | FALSE |
only showing the first 7 rows
Run gl.drop.sexlinked
Remove the sex-linked loci from the LBP genlight object:
new.gl <- gl.drop.sexlinked(LBP, system = "xy")new.gl
new.gl@n.loc![]() How many SNPs are there left after removing sex-linked loci?
How many SNPs are there left after removing sex-linked loci?
More filtering
Knock yourself out trying all the different report and filtering functions
Remember to use them as pairs – report function to decide a threshold, filter function to apply the threshold (where appropriate)
Below are two tables with a number of report and filter functions you can try.
Report functions
| Report functions | description |
|---|---|
| gl.report.callrate | summarises CallRate values |
| gl.report.reproducibility | summarises repAvg (SNP) or reproducibility (SilicoDArT) values. |
| gl. report.monomorphs | provides a count of polymorphic and monomorphic loci |
| gl.report.secondaries | provides a count of loci that are secondaries, that is, loci that reside on the one sequence tag. |
| gl.report.rdepth | reports the estimate of average read depth for each locus |
| gl.report.hamming | reports on Hamming distances between sequence tags |
| gl.report.overshoot | reports loci for which the SNP has been trimmed along with the adaptor sequence |
| gl.report.taglength | reports a frequency tabulation of sequence tag lengths |
| gl.report.sexlinked | reports the number and type of loci likely to be in sex chromosomes |
Filtering functions
| Filter functions | description |
|---|---|
| gl.filter.callrate | filter out loci or individuals for which the call rate (rate of non-missing values) is less than a specified threshold, say threshold = 0.95 |
| gl.filter.reproducibility | filter out loci for which the reproducibility (strictly repeatability) is less than a specified threshold, say threshold = 0.99 |
| gl. filter.monomorphs | provides a count of polymorphic and monomorphic loci |
| gl.filter.allna | filter out loci that are all missing values (NA) |
| gl.filter.secondaries | filter out SNPs that share a sequence tag, except one retained at random [or the best based on reproducibility (RepAvg) and information content (AvgPIC)]. |
| gl.filter.rdepth | filter out loci with exceptionally low or high read depth (coverage) |
| gl.filter.hamming | filter out loci that differ from each other by less than a specified number of base pairs |
| gl.filter.overshoot | filter out loci where the SNP location lies outside the trimmed sequence tag |
| gl.filter.taglength | filter out loci for which the tag length is less that a threshold |
| gl.filter.sexlinked | filter out loci that are likely to be in sex chromosomes |
How about trying it with a different dataset?
# how to load a genlight object
gl <- gl.read.dart(filename="./data/sample_data_2row.csv",
ind.metafile="./data/sample_metadata.csv")You can even try it with your own data.
Further Study
For more tuturials see the dartR Tutorials section.
Check out the other functions contained in package dartR.sexlinked. What do they do?
Keep your eyes peeled for Session 8!
Readings
Gruber et al. (2018)
Mijangos et al. (2022)
Jombart and Ahmed (2011)
Robledo-Ruiz et al. (2023)