#BiocManager::install("LEA")
#devtools::install_github("tdhock/directlabels")
library(dartRverse)
library(ggplot2)
library(data.table)
library(leaflet.minicharts)
library(LEA)2 Pop Gen In Conservation
Session Presenters

Required packages
make sure you have the packages installed, see Install dartRverse
Introduction
Here is an introductory video on genetic diversity measures that is assumed knowledge for this session.
For more details see Sherwin et al. (2017).
Exercise
![]() You have been asked to evaluate the ‘genetic health’ of a population at a restoration site, which has been established two years earlier as a results of the drying of a wet land due to urban development. The corporate responsible for the restoration project claims that the area can now sustain a high density population of the species A, which previously only occurred in the forested areas west and east of the previously existing wet land. Species A is a long lived terrestrial animal.
You have been asked to evaluate the ‘genetic health’ of a population at a restoration site, which has been established two years earlier as a results of the drying of a wet land due to urban development. The corporate responsible for the restoration project claims that the area can now sustain a high density population of the species A, which previously only occurred in the forested areas west and east of the previously existing wet land. Species A is a long lived terrestrial animal.
Compute genetic diversity metrics (e.g. \(He\), \(Ho\), \(Fis\)) with the data generated by the 100 samples collected and provide a recommendation on whether the restoration project was successful and whether the studied population is likely to required continued active management in the short term.
Load data
# Load the data
glsim <- gl.load("./data/sess2_glsim.rds")#check the population size and number of populations
table(pop(glsim))
popA
100 #number of loci
nLoc(glsim)[1] 2500Different dynamics, same result
Several descriptive genetic parameters are almost routinely computed, and one would be tempted to interpret them with a standardised approach. For example, low genetic diversity is often considered as an indication that the population size is small. If the inbreeding coefficient (\(Fis\)) is positive, that is often taken as an indication that the population is inbred and potentially at risk of inbreeding depression. However, there are situation where different dynamics can produce the same result. It is not always easy to diagnose these situations and identify the cause of our results, but my recommendation would be to give priority to your understanding of the biology, ecology and history of the populations and species you are working with. If the suggested interpretation do not fit your understanding of the ecology or demographics of the populations you are working with, explore possible alternative explanations. In the example above, a low genetic diversity can also occur because of a severe bottleneck even if the population has then recovered to large numbers.
Recall that \(Fis = (He-Ho)/He\), hence any cause of alteration of HWE can cause an increased/decrease \(Fis\) as well sampling effects. For example, the selective sampling of related individuals (e.g. when sampling individuals within family groups - as often happens with feral pigs/boar - or when you are sampling intensively within the dispersal distance range of few individuals) can cause your samples not to be representative of the population, and while your analysis could indicate inbreeding, this may not be the case at population level. Wahlund effect is also another common cause of increased \(Fis\).
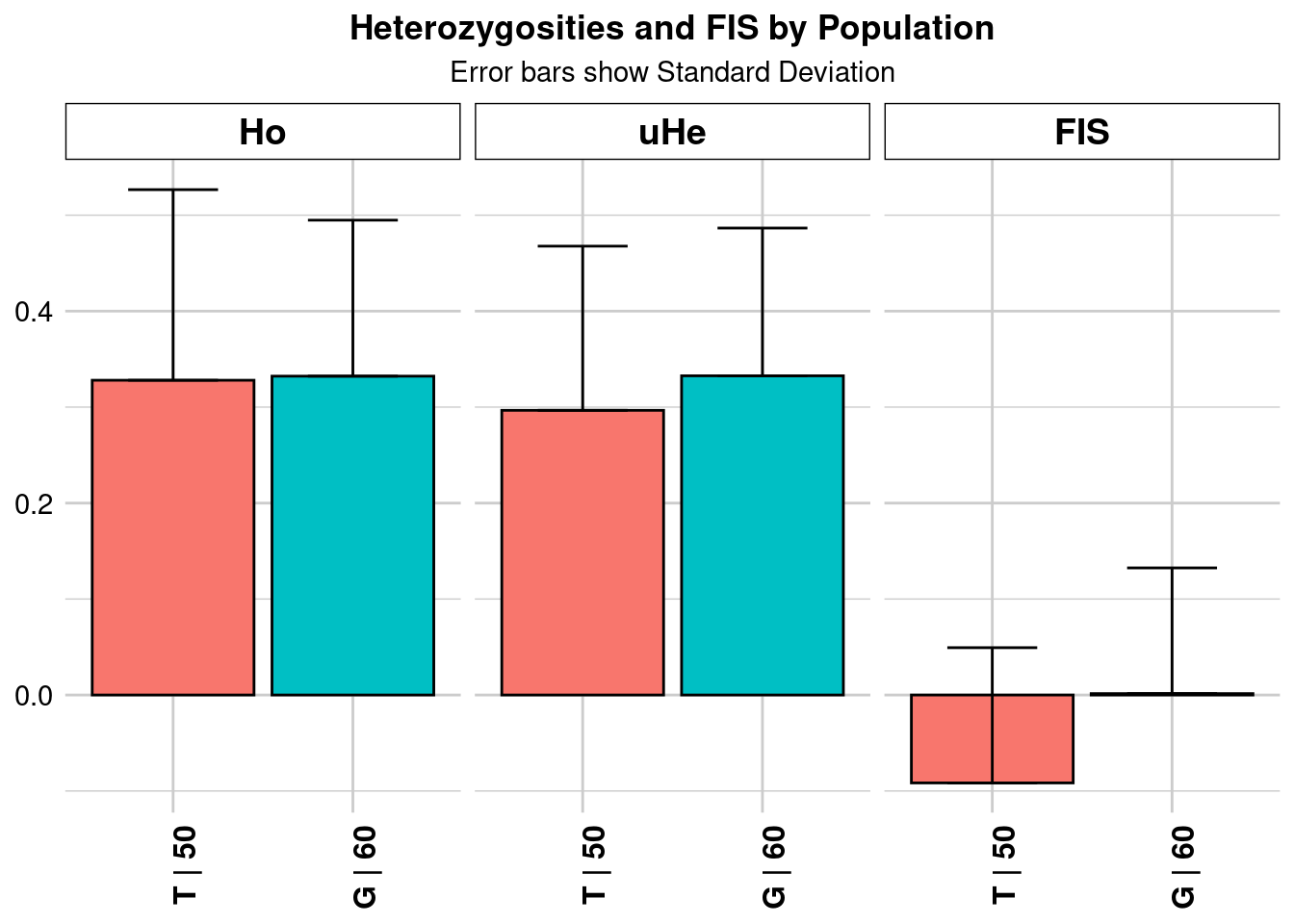
Let’s have a look at an example of what happens if our sampling design caused us to sample selectively related individuals. We are going to use a simulated dataset based on the concept presented in Pacioni et al (2020) where females intensively trapped (using a grid design, ‘G’) within an area that didn’t encompassed multiple home ranges were highly related. ON the contrary, individuals trapped along transect (‘T’) that extended over multiple home ranges were an actual random rapresentation of the population.
Load data
glw <- gl.load("./data/sess2_glDes.rds")
#check the population size and number of populations
table(pop(glw)) # G stands for trapped in a grid, T for transect
#number of loci
nLoc(glw)
glw <- gl.filter.monomorphs(glw, verbose = 5)Exercise data
gl.report.heterozygosity(glsim)Starting gl.report.heterozygosity
Processing genlight object with SNP data
Calculating Observed Heterozygosities, averaged across
loci, for each population
Calculating Expected Heterozygosities
Starting gl.colors
Selected color type dis
Completed: gl.colors 
pop n.Ind n.Loc n.Loc.adj polyLoc monoLoc all_NALoc Ho HoSD
popA popA 100 2500 1 2500 0 0 0.386004 0.142553
HoSE HoLCI HoHCI Ho.adj Ho.adjSD Ho.adjSE Ho.adjLCI Ho.adjHCI
popA 0.002851 NA NA 0.386004 0.142553 0.002851 NA NA
He HeSD HeSE HeLCI HeHCI uHe uHeSD uHeSE uHeLCI
popA 0.441149 0.096207 0.001924 NA NA 0.443366 0.09669 0.001934 NA
uHeHCI He.adj He.adjSD He.adjSE He.adjLCI He.adjHCI FIS FISSD
popA NA 0.441149 0.096207 0.001924 NA NA 0.114365 0.268448
FISSE FISLCI FISHCI
popA 0.005369 NA NA
Completed: gl.report.heterozygosity pcaglsim <- gl.pcoa(glsim)Starting gl.pcoa
Processing genlight object with SNP data
Performing a PCA, individuals as entities, loci as attributes, SNP genotype as state
Starting gl.colors
Selected color type 2
Completed: gl.colors 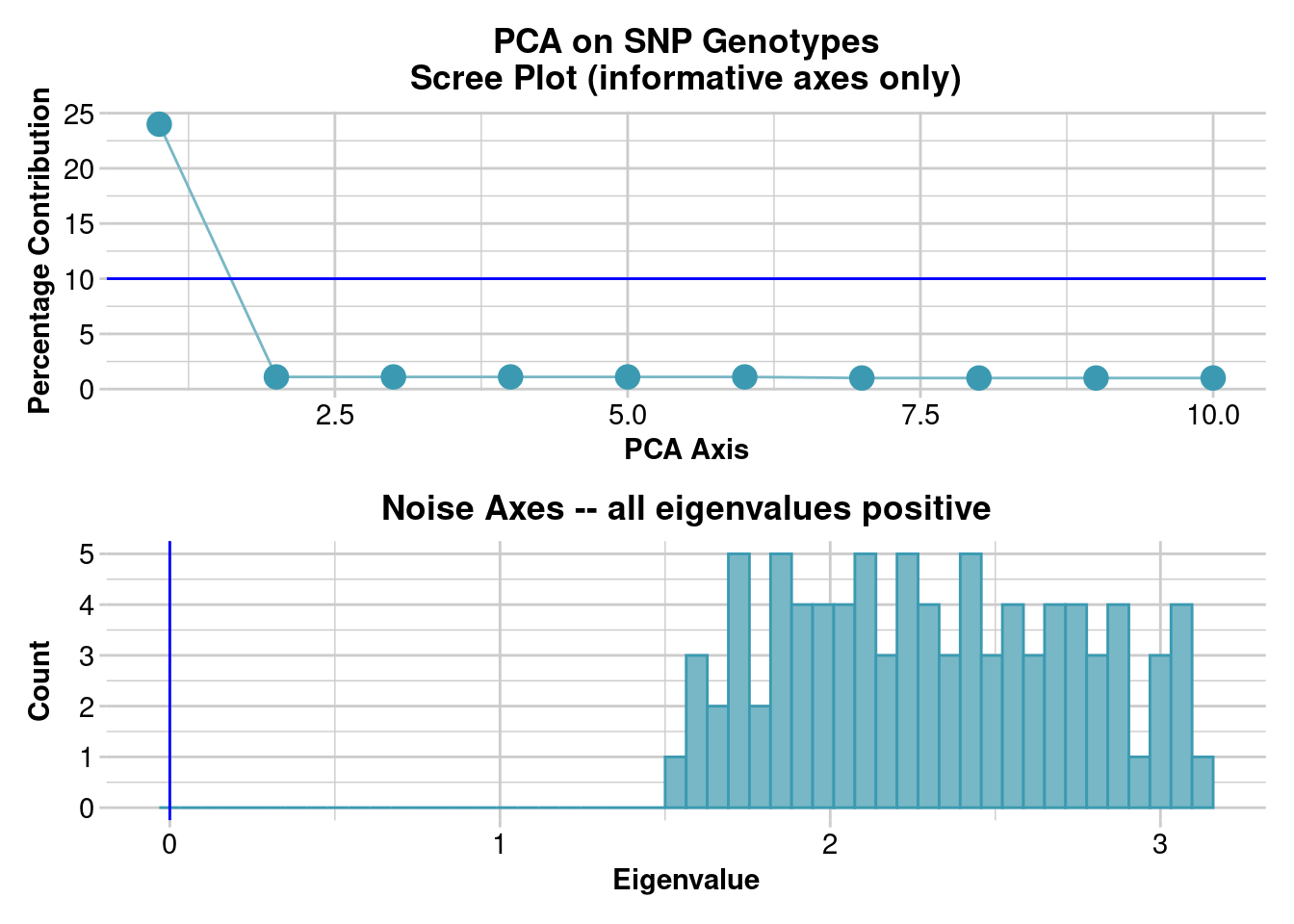
Completed: gl.pcoa gl.pcoa.plot(glPca = pcaglsim, x = glsim)Starting gl.pcoa.plot
Processing an ordination file (glPca)
Processing genlight object with SNP data
Package directlabels needed for this function to work. Please install it.[1] -1hwe <- gl.report.hwe(glsim, multi_comp = TRUE)Starting gl.report.hwe
Processing genlight object with SNP data
Package HardyWeinberg needed for this function to work. Please install it.Assessing Populations: structure and demographic history
Load data and explore
This dataset is to assess population structure and diversity in Uperoleia crassa, from Jaya et al. (2022).
# gl <- dartR::gl.read.dart("Report_DUp20-4995_1_moreOrders_SNP_mapping_2.csv",
# ind.metafile = "Uperoleia_metadata.csv")
load('./data/session_2.RData') # data named glThese are monsoonal tropical frogs, who are very common and abundant. They do evolutionarily and reproductively interesting and weird stuff.
Lets quickly look at our samples and populations.
gl.map.interactive(gl)Starting gl.map.interactive
Processing genlight object with SNP data
Completed: gl.map.interactive We have two populations - the Kimberley (IK) and the Top End (IT)
Clean data
Clean up your dataset to remove the most egregiously bad loci and individuals this set of filtering can be used across analyses,
#Get rid of really poorly sequenced loci
#But don’t cut hard
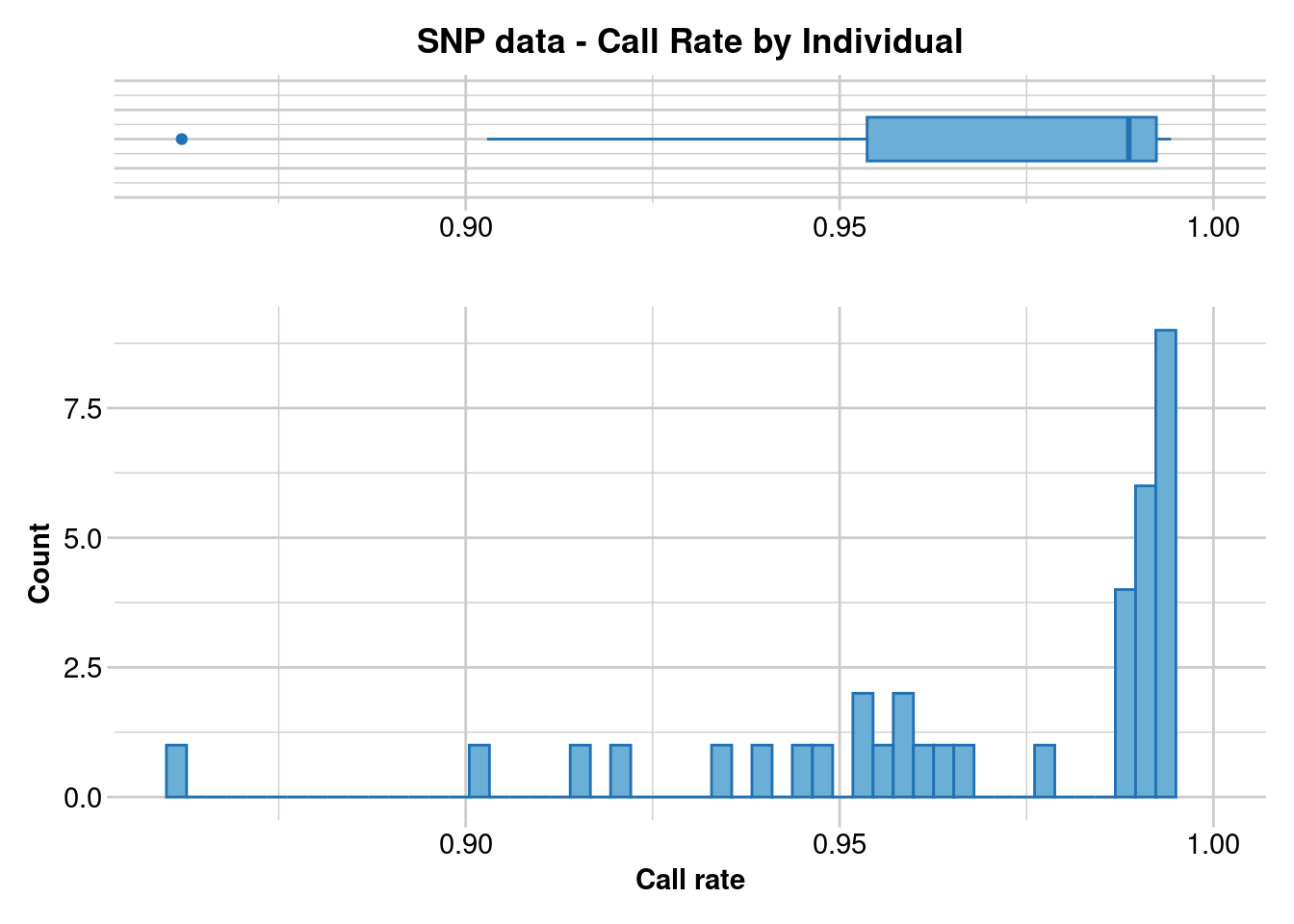
gl.report.callrate(gl)Starting gl.report.callrate
Processing genlight object with SNP data
Reporting Call Rate by Locus
No. of loci = 227143
No. of individuals = 36
Minimum : 0.222222
1st quartile : 0.611111
Median : 0.777778
Mean : 0.7464089
3r quartile : 0.916667
Maximum : 1
Missing Rate Overall: 0.2536 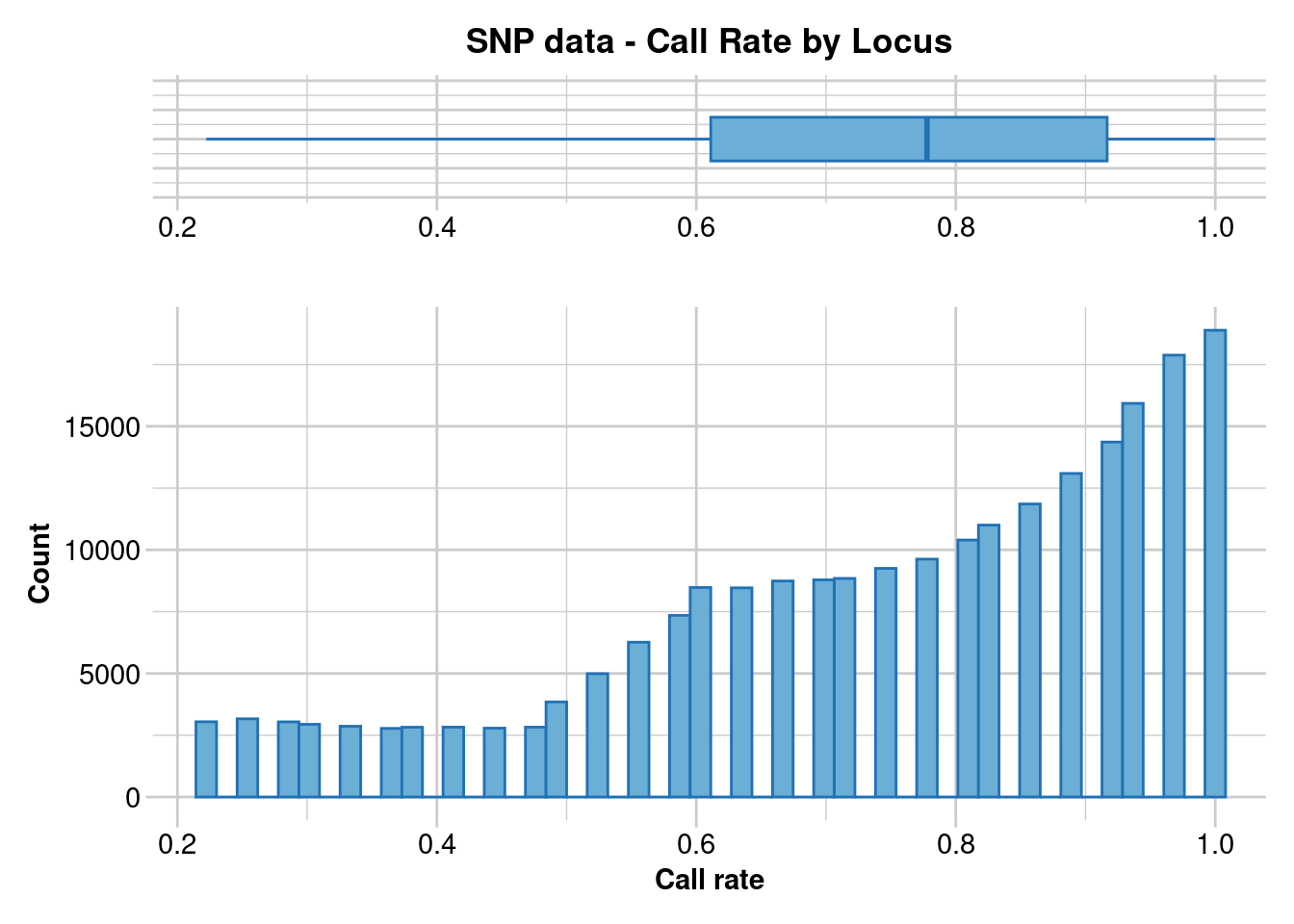
Completed: gl.report.callrate gl.1 <- gl.filter.callrate(gl, method = "loc", threshold = 0.8)Starting gl.filter.callrate
Processing genlight object with SNP data
Warning: Data may include monomorphic loci in call rate
calculations for filtering
Recalculating Call Rate
Removing loci based on Call Rate, threshold = 0.8 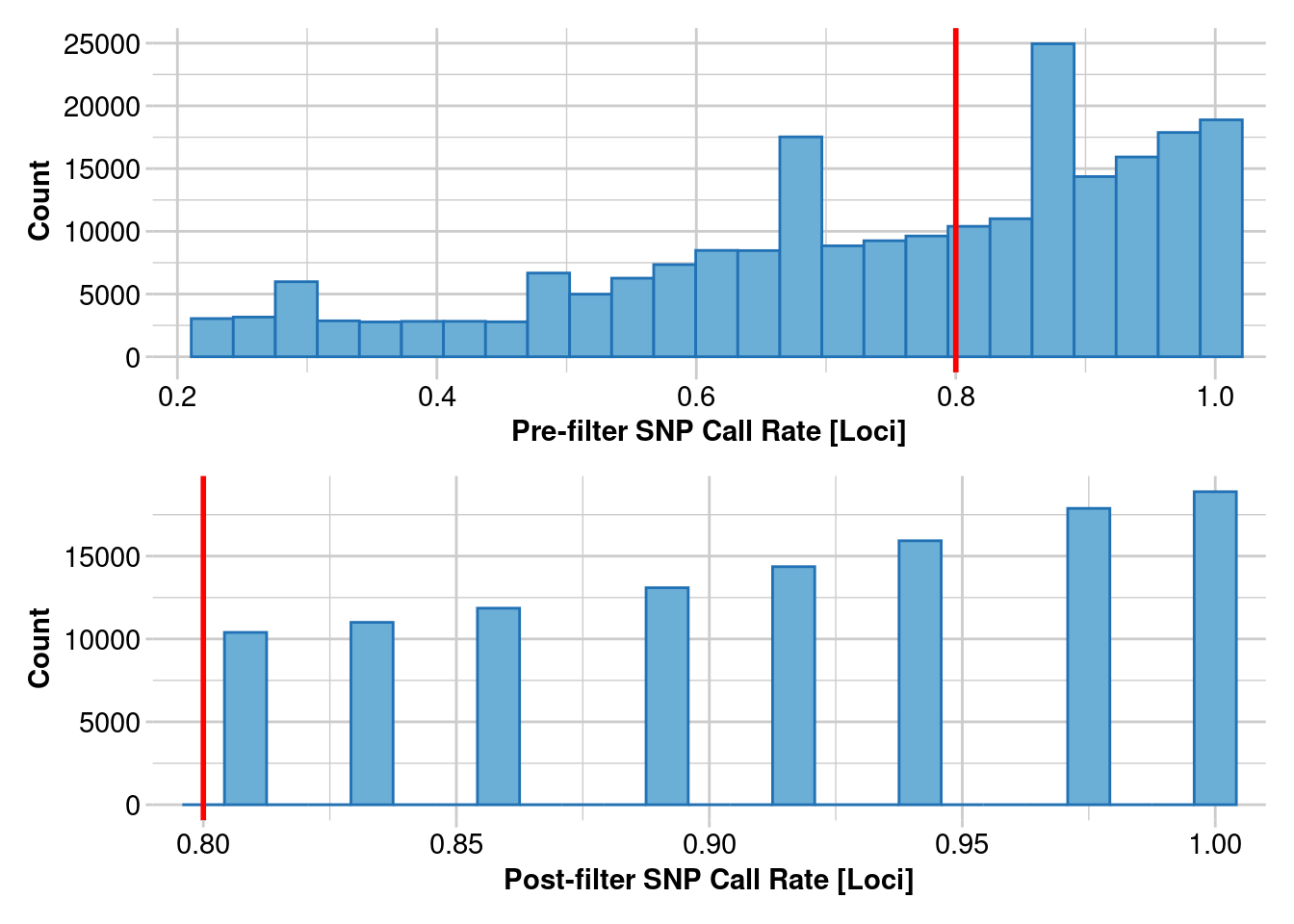
Completed: gl.filter.callrate #Very low filter – this is only to get rid of your really bad individuals
gl.report.callrate(gl.1, method = "ind")Starting gl.report.callrate
Processing genlight object with SNP data
Reporting Call Rate by Individual
No. of loci = 113404
No. of individuals = 36
Minimum : 0.7589944
1st quartile : 0.8751808
Median : 0.9524179
Mean : 0.9159171
3r quartile : 0.9598625
Maximum : 0.9787662
Missing Rate Overall: 0.0841
Listing 2 populations and their average CallRates
Monitor again after filtering
Population CallRate N
1 IK 0.9567 22
2 IT 0.8519 14
Listing 20 individuals with the lowest CallRates
Use this list to see which individuals will be lost on filtering by individual
Set ind.to.list parameter to see more individuals
Individual CallRate
1 Up1041_R138706 0.7589944
2 Up1186_QMJ88804 0.7730856
3 Up1096_R36195 0.8080932
4 Up0969_ABTC29129 0.8429597
5 Up1136_NTMR36228 0.8449878
6 Up0854_ABTC17229 0.8483299
7 Up0276_NTMR35114 0.8558869
8 Up1118_NTMR36173 0.8596699
9 Up1120_R36170 0.8701809
10 Up0767_ABTC12489 0.8768474
11 Up0731_ABTC99709 0.8850834
12 Up0832_ABTC93392 0.8948097
13 Up0769_ABTC12511 0.8980988
14 Up0166_WAMR162490 0.9082308
15 Up1078_R36177 0.9091390
16 Up1269_WAMR164857 0.9188212
17 Up1266_WAMR164844 0.9397640
18 Up1321_WAMR171536 0.9517389
19 WAMR162566 0.9530969
20 Up0083_WAMR113846 0.9537142
)
Completed: gl.report.callrate gl.2 <- gl.filter.callrate(gl.1, method = "ind", threshold = 0.25)Starting gl.filter.callrate
Processing genlight object with SNP data
Warning: Data may include monomorphic loci in call rate
calculations for filtering
Recalculating Call Rate
Removing individuals based on Call Rate, threshold = 0.25 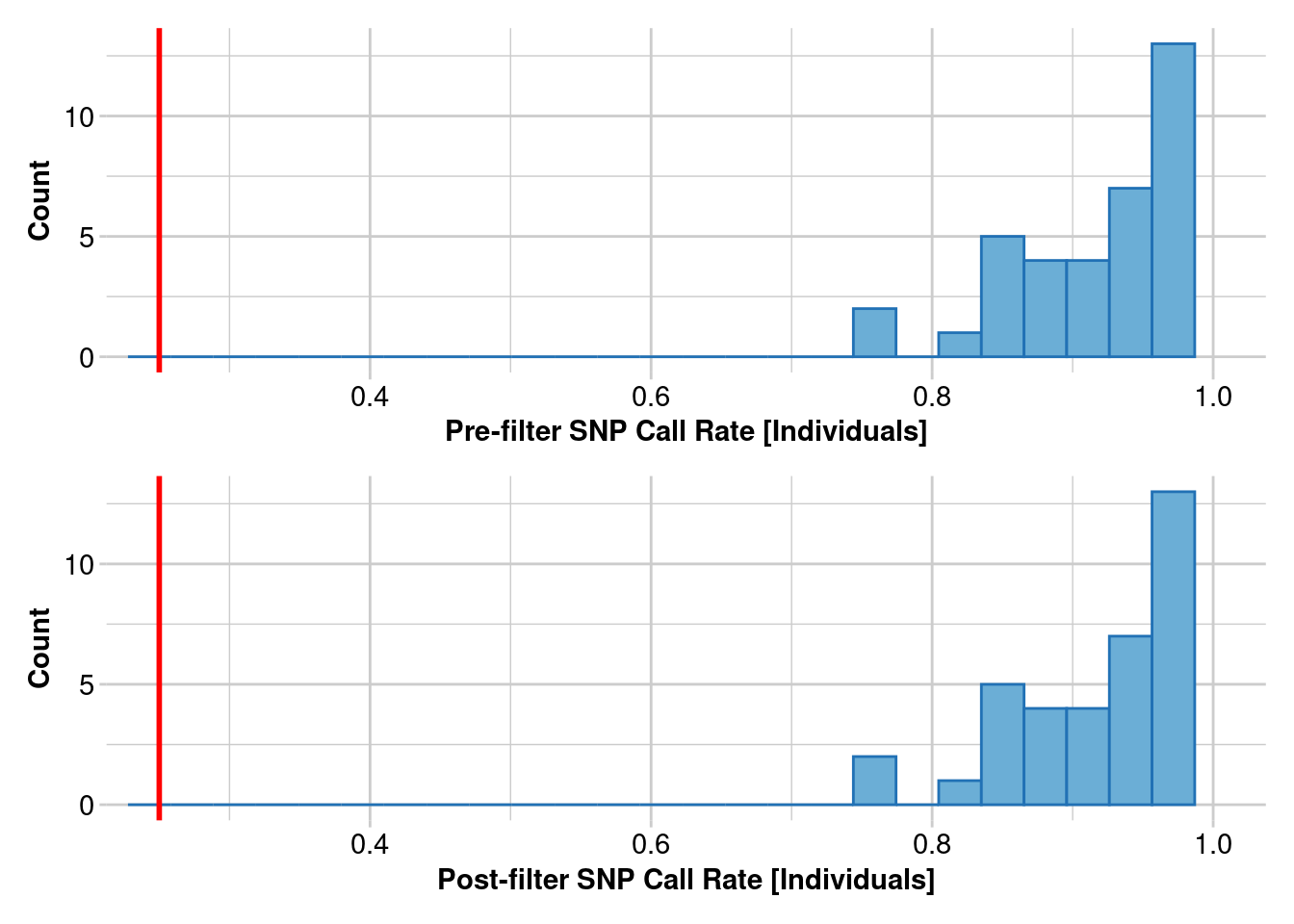
Note: Locus metrics not recalculated
Note: Resultant monomorphic loci not deleted
Completed: gl.filter.callrate #Always run this after removing individuals – removes loci that are no longer variable
gl.3 <- gl.filter.monomorphs(gl.2)Starting gl.filter.monomorphs
Processing genlight object with SNP data
Identifying monomorphic loci
No monomorphic loci to remove
Completed: gl.filter.monomorphs #Get rid of unreliable loci
gl.report.reproducibility(gl.3)Starting gl.report.reproducibility
Processing genlight object with SNP data
Reporting Repeatability by Locus
No. of loci = 113404
No. of individuals = 36
Minimum : 0.875
1st quartile : 1
Median : 1
Mean : 0.9962267
3r quartile : 1
Maximum : 1
Missing Rate Overall: 0.08 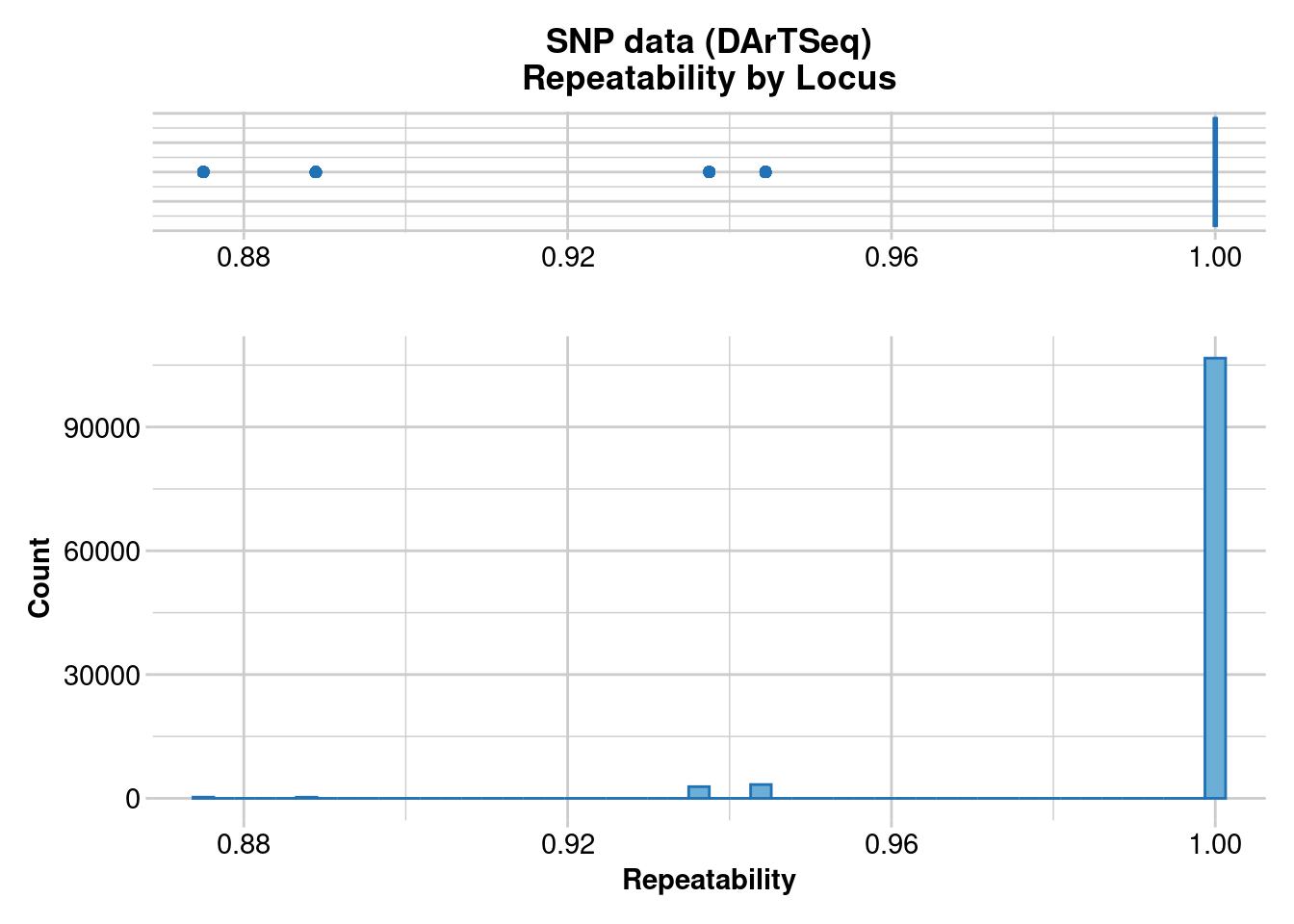
Quantile Threshold Retained Percent Filtered Percent
1 100% 1.000000 106676 94.1 6728 5.9
2 95% 1.000000 106676 94.1 6728 5.9
3 90% 1.000000 106676 94.1 6728 5.9
4 85% 1.000000 106676 94.1 6728 5.9
5 80% 1.000000 106676 94.1 6728 5.9
6 75% 1.000000 106676 94.1 6728 5.9
7 70% 1.000000 106676 94.1 6728 5.9
8 65% 1.000000 106676 94.1 6728 5.9
9 60% 1.000000 106676 94.1 6728 5.9
10 55% 1.000000 106676 94.1 6728 5.9
11 50% 1.000000 106676 94.1 6728 5.9
12 45% 1.000000 106676 94.1 6728 5.9
13 40% 1.000000 106676 94.1 6728 5.9
14 35% 1.000000 106676 94.1 6728 5.9
15 30% 1.000000 106676 94.1 6728 5.9
16 25% 1.000000 106676 94.1 6728 5.9
17 20% 1.000000 106676 94.1 6728 5.9
18 15% 1.000000 106676 94.1 6728 5.9
19 10% 1.000000 106676 94.1 6728 5.9
20 5% 0.944444 110021 97.0 3383 3.0
21 0% 0.875000 113404 100.0 0 0.0
Completed: gl.report.reproducibility gl.4 <- gl.filter.reproducibility(gl.3)Starting gl.select.colors
Warning: Number of required colors not specified, set to 9
Library: RColorBrewer
Palette: brewer.pal
Showing and returning 2 of 9 colors for library RColorBrewer : palette Blues 
Completed: gl.select.colors
Starting gl.filter.reproducibility
Processing genlight object with SNP data
Removing loci with repeatability less than 0.99 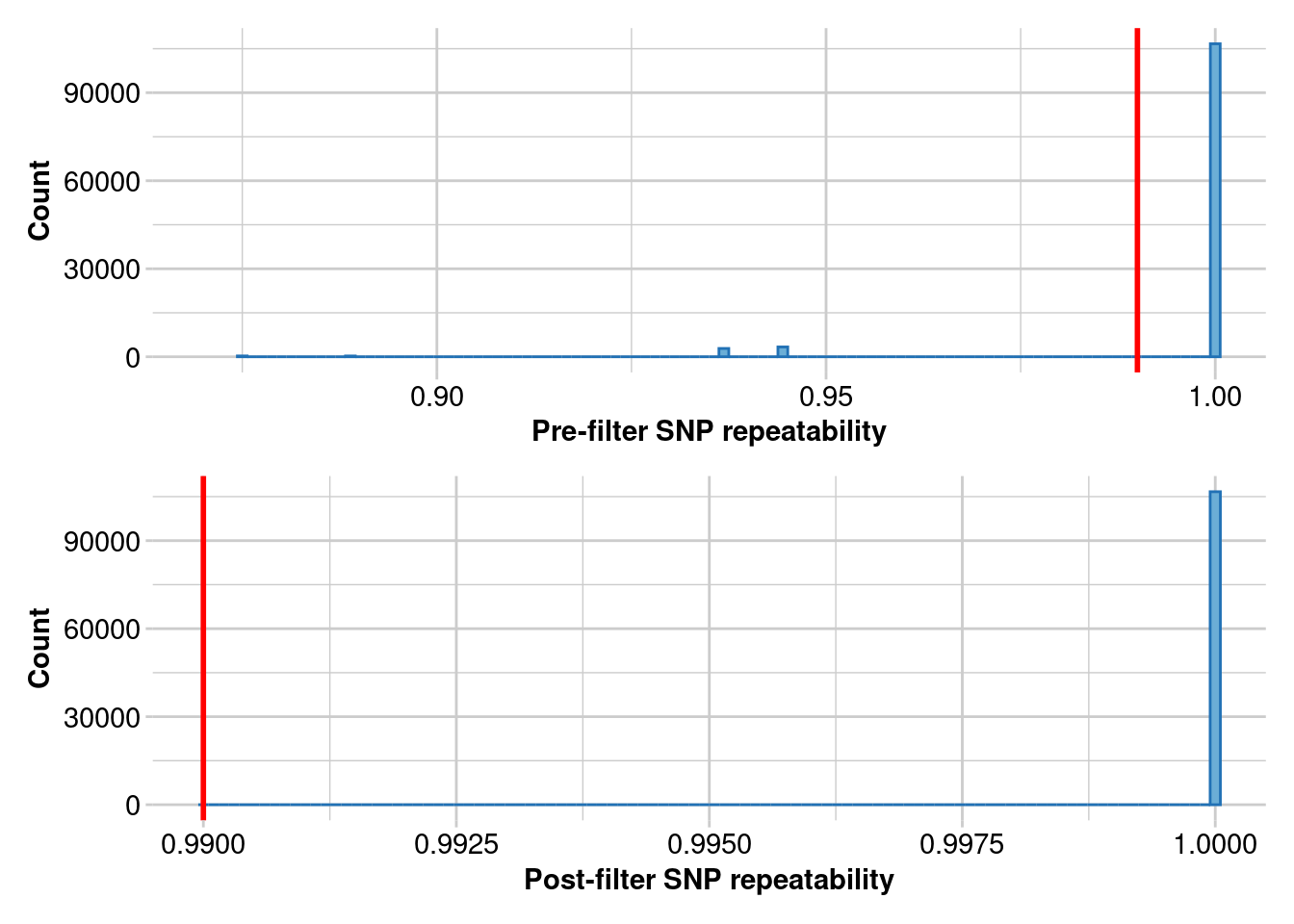
Completed: gl.filter.reproducibility #Get rid of low and super high read depth loci
#do twice so you can zoom in
gl.report.rdepth(gl.4)Starting gl.report.rdepth
Processing genlight object with SNP data
Reporting Read Depth by Locus
No. of loci = 106676
No. of individuals = 36
Minimum : 2.5
1st quartile : 5
Median : 7.2
Mean : 7.741356
3r quartile : 10
Maximum : 121.7
Missing Rate Overall: 0.09 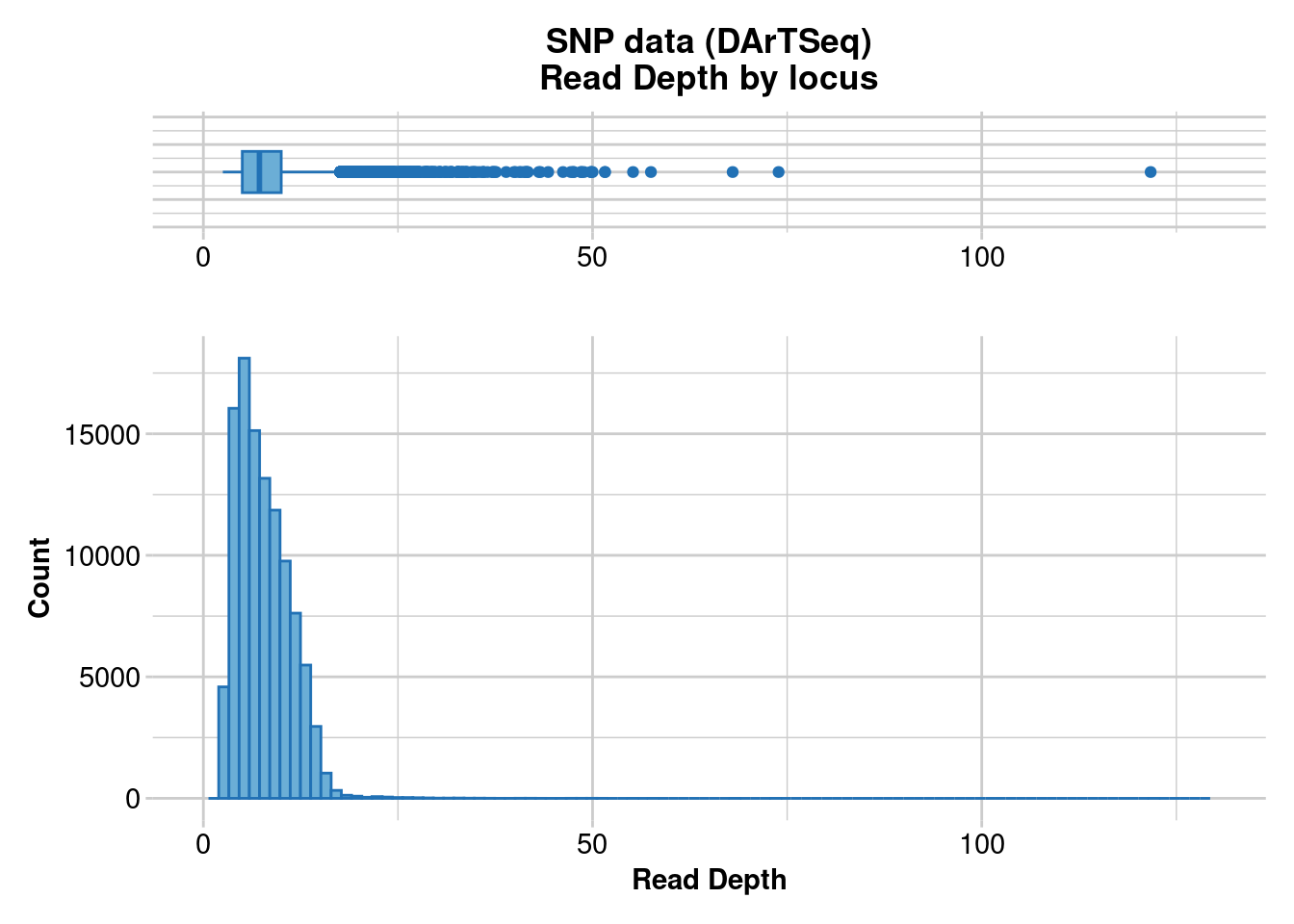
Quantile Threshold Retained Percent Filtered Percent
1 100% 121.7 1 0.0 106675 100.0
2 95% 13.6 5520 5.2 101156 94.8
3 90% 12.4 10872 10.2 95804 89.8
4 85% 11.5 16057 15.1 90619 84.9
5 80% 10.7 21633 20.3 85043 79.7
6 75% 10.0 26932 25.2 79744 74.8
7 70% 9.4 32193 30.2 74483 69.8
8 65% 8.8 37650 35.3 69026 64.7
9 60% 8.2 43461 40.7 63215 59.3
10 55% 7.7 48582 45.5 58094 54.5
11 50% 7.2 53911 50.5 52765 49.5
12 45% 6.7 59668 55.9 47008 44.1
13 40% 6.3 64353 60.3 42323 39.7
14 35% 5.8 70391 66.0 36285 34.0
15 30% 5.4 75457 70.7 31219 29.3
16 25% 5.0 80644 75.6 26032 24.4
17 20% 4.6 86041 80.7 20635 19.3
18 15% 4.2 91353 85.6 15323 14.4
19 10% 3.8 96433 90.4 10243 9.6
20 5% 3.3 102090 95.7 4586 4.3
21 0% 2.5 106676 100.0 0 0.0
Completed: gl.report.rdepth gl.5 <- gl.filter.rdepth(gl.4, lower = 0, upper = 25)Starting gl.filter.rdepth
Processing genlight object with SNP data
Removing loci with rdepth <= 0 and >= 25 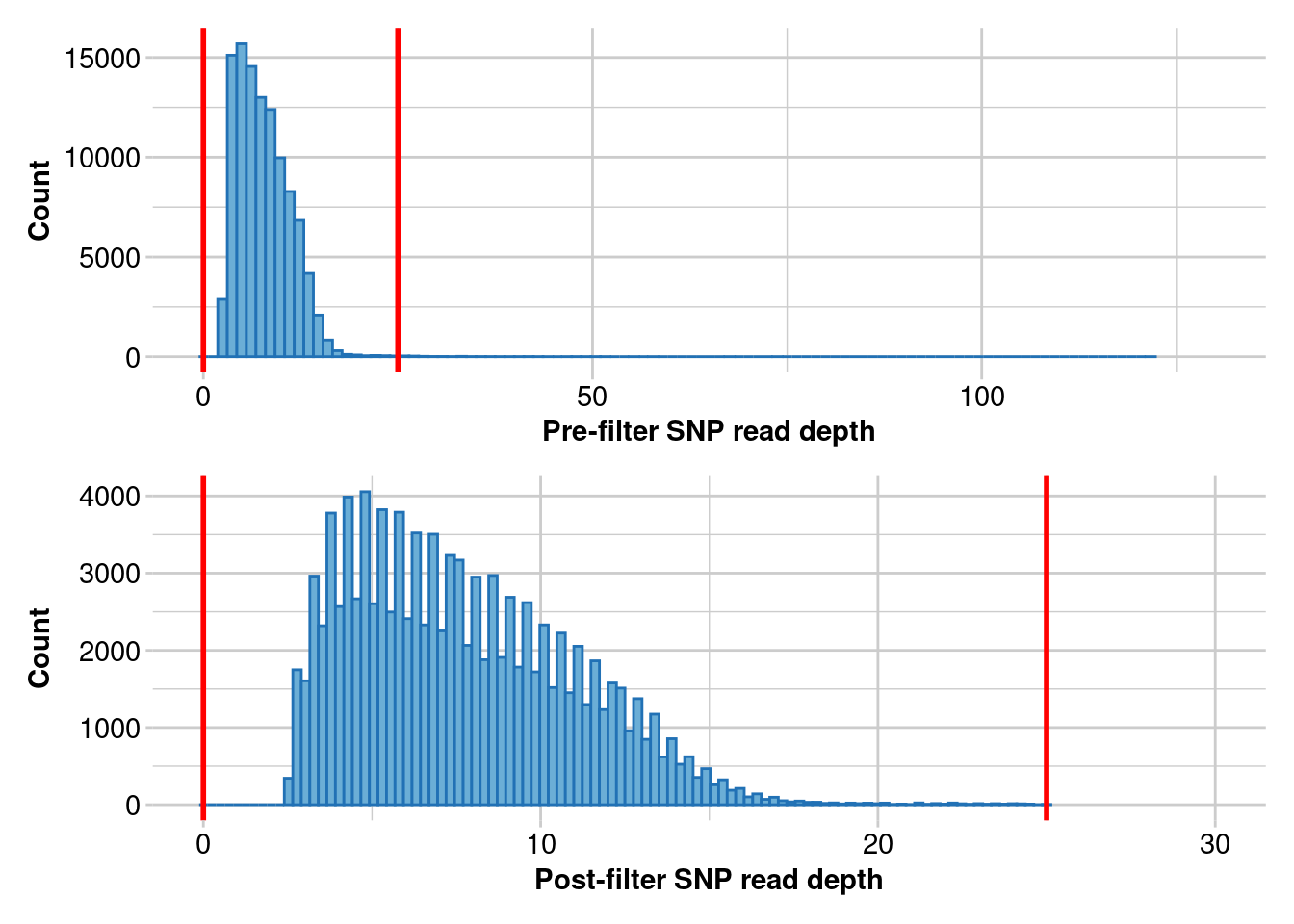
Completed: gl.filter.rdepth gl.clean <- gl.filter.rdepth(gl.5, lower = 8, upper = 17)Starting gl.filter.rdepth
Processing genlight object with SNP data
Removing loci with rdepth <= 8 and >= 17 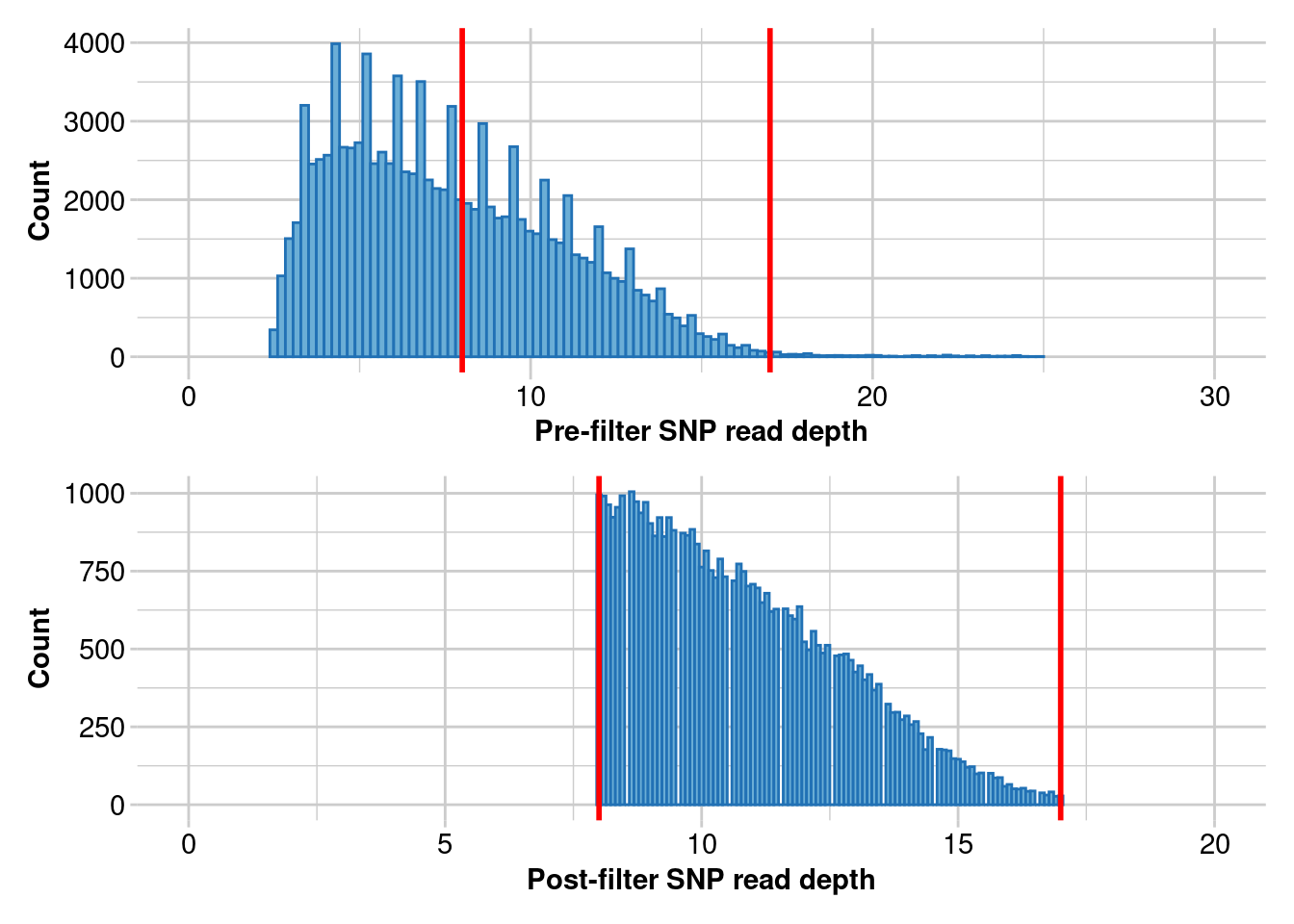
Completed: gl.filter.rdepth nInd(gl.clean)[1] 36nLoc(gl.clean)[1] 44752#look at the data to see if you see any obvious issues and redo if you do.
plot(gl.clean)
rm(gl.1, gl.2, gl.3, gl.4, gl.5)This file is now your starting file for other filtering
Filtering
Filtering data for population structure and phylogenetic analyses
Loci on the same fragment and missing data are not well supported in these analyses, as they expect all loci to be independently inherited. This means very very close loci are not going to be split through recombination and should be removed. Structure-like analyses dislike missing data. We are also going to filter harder than normal here just so that we can see a result within the workshop.
gl.report.callrate(gl.clean)gl.1 <- gl.filter.callrate(gl.clean, method = "loc", threshold = 0.98)#Remove minor alleles
#I usually set up the threshold so it is just
# removing singletons to improve computation time
gl.report.maf(gl.1)gl.2 <- gl.filter.maf(gl.1, threshold = 1/(2*nInd(gl.1)))#check that the data looks fairly clean
#this starts to show some obvious population banding
plot(gl.2)#remove secondary SNPs on the same fragment
#Always do this as the last loci filter so that you’ve cut for quality
# before you cut because there are two SNPs
gl.3 <- gl.filter.secondaries(gl.2)
#Filter on individuals. You can usually be a bit flexible at this point.
#individuals look a whole lot better now
#make note of any idnviduals with a low call rate. Keep them in for now
#but if they act weird in the analysis, you may want to consider removing
gl.report.callrate(gl.3, method = "ind")gl.4 <- gl.filter.callrate(gl.3, method = "ind", threshold = .9)#Always run this after removing individuals
gl.structure <- gl.filter.monomorphs(gl.4)
#this is your cleaned dataset for a population structure analysis
plot(gl.structure)nInd(gl.structure)
nLoc(gl.structure)You can write this file out for various analyses that we will not go into here (e.g. gl2structure(x) or gl2faststructure(x))
Run SNMF (LEA)
This is a structure-like analysis called SNMF. This code is not all you would need to publish this result, but it is a good first look. You need to test your parameter settings (alpha and tolerance) in a real analysis.
#takes quite a while to run (about 15 minutes)
#in case you want to run it uncomment the following lines and comment the readRDS line
# LEA requires the genotype style file
gl2faststructure(gl.structure, outfile = "gl_structure.fstr",
outpath = './data/')
struct2geno("./data/gl_structure.fstr", ploidy = 2, FORMAT = 2)
###this hates any loci with all heterozygotes
snmf.Sy.K1_8.10 = snmf("./data/gl_structure.fstr.geno", K = 1:8,
entropy = T, ploidy = 2, project="new", repetitions = 10)plot(snmf.Sy.K1_8.10)
k <- 2 #chose best based on lowest cross entropy in graph
ce = cross.entropy(snmf.Sy.K1_8.10, K = k)
best <- which.min(ce)
par (mfrow = c(1,1))
barplot(t(Q(snmf.Sy.K1_8.10, K = k, run = best)), col = 1:k)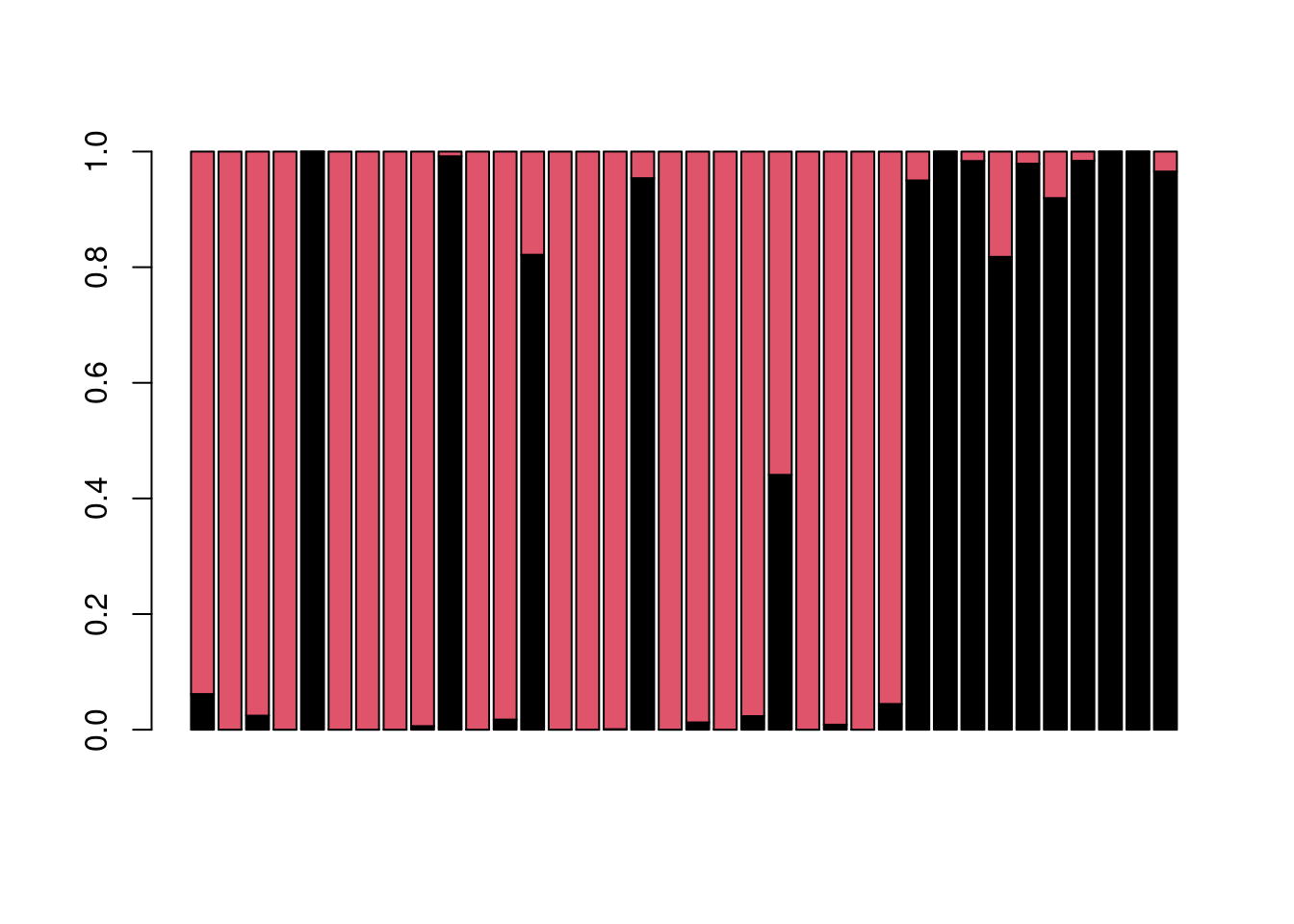
#Do a PCoA plot. I hate these but they are also good for first pass visualisation.
pc <- gl.pcoa(gl.structure)Starting gl.pcoa
Processing genlight object with SNP data
Performing a PCA, individuals as entities, loci as attributes, SNP genotype as state
Starting gl.colors
Selected color type 2
Completed: gl.colors 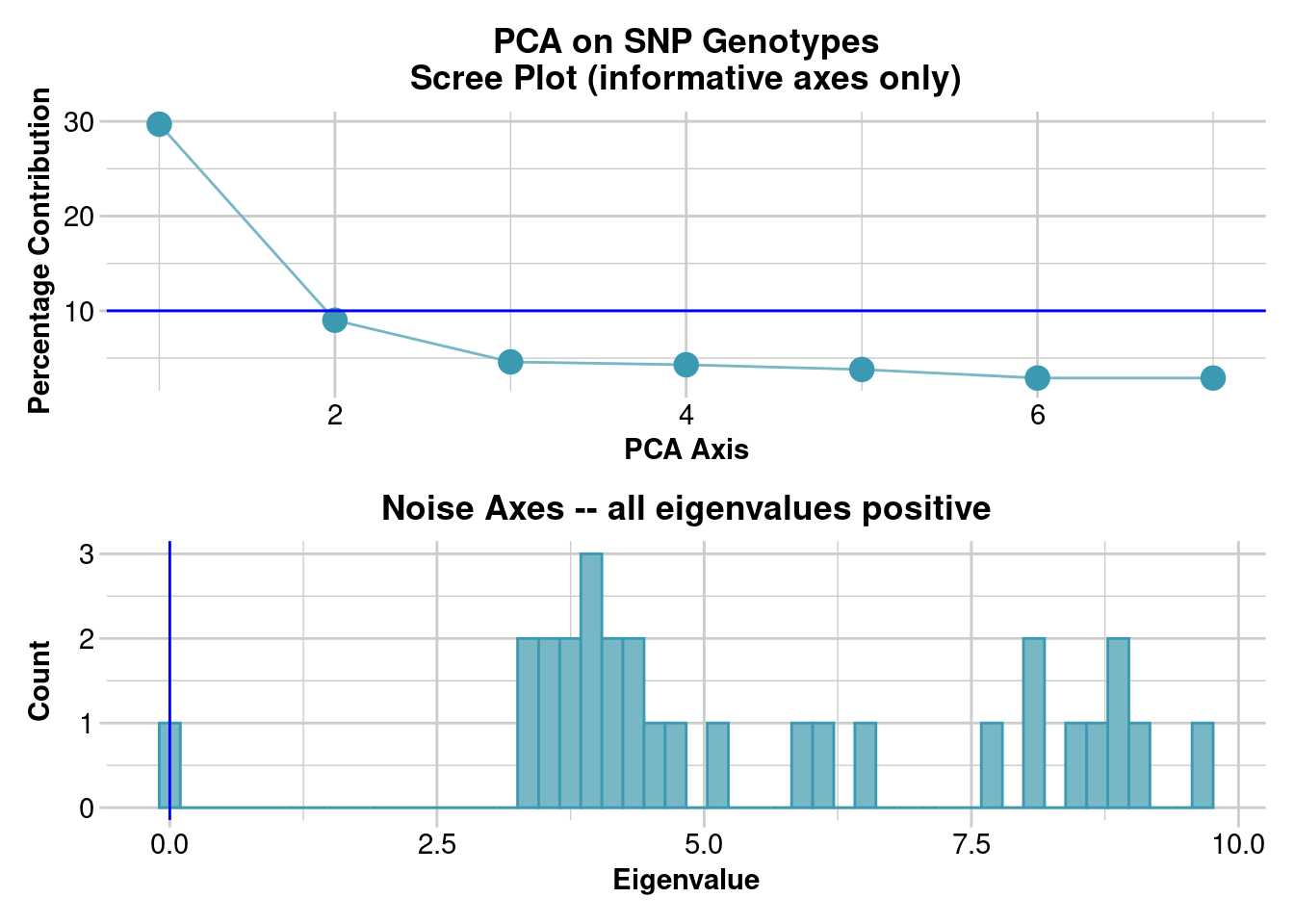
Completed: gl.pcoa gl.pcoa.plot(pc, gl.structure)Starting gl.pcoa.plot
Processing an ordination file (glPca)
Processing genlight object with SNP data
Package directlabels needed for this function to work. Please install it.[1] -1rm(ce, gl.1, gl.2, gl.3, gl.4, snmf.Sy.K1_8.10, best, k, pc)![]() Have a go at altering various parameters and seeing how this changes your answers.
Have a go at altering various parameters and seeing how this changes your answers.
One thing that regularly changes with MAF filters is the amount of variance explained. Removing more minor alleles increases the variance explained in a PCoA. Think about why that would be the case.
Filtering for Tajima’s D
Now we are going to look at how filtering can affect your understanding of demographic processes that population is undergoing. In this case we are going to look at the metric Tajima’s D. Significant negative values of Tajima’s D are due to an excess of rare alleles and are consistent with range expansion. Significant positive values are associated with population contraction.
The moral of the story here is that rare alleles matter, especially when looking at population demographics! Lets look at how singletons impact our estimations of whether a population is expanding or contracting.
This is a function written to calculate Tajima’s D from SNP data. This code will create the function in your global environment so that you can call it. This function differs from calculations in packages such as heirfstat and popgen because it deals with missing data correctly for SNPs, while those other programs were built for gene alignments.Significance (a P-value) cannot be calculated without a simulation, and we are not doing that here. Please ask me how to do this using Hudson’s ms program if you are interested.
get_tajima_D <- function(x){
#require(dartRverse) # possibly not needed for a function in an R package?
# Find allele frequencies (p1 and p2) for every locus in every population
allele_freqs <- dartR::gl.percent.freq(x)
names(allele_freqs)[names(allele_freqs) == "frequency"] <- "p1"
allele_freqs$p1 <- allele_freqs$p1 / 100
allele_freqs$p2 <- 1 - allele_freqs$p1
# Get the names of all the populations
pops <- unique(allele_freqs$popn)
#split each population
allele_freqs_by_pop <- split(allele_freqs, allele_freqs$popn)
# Internal function to calculate pi
calc_pi <- function(allele_freqs) {
n = allele_freqs$nobs * 2 # vector of n values
pi_sqr <- allele_freqs$p1 ^ 2 + allele_freqs$p2 ^ 2
h = (n / (n - 1)) * (1 - pi_sqr) # vector of values of h
sum(h) # return pi, which is the sum of h across loci
}
get_tajima_D_for_one_pop <- function(allele_freqs_by_pop) {
pi <- calc_pi(allele_freqs_by_pop)
# Calculate number of segregating sites, ignoring missing data (missing data will not appear in teh allele freq calcualtions)
#S <- sum(!(allele_freqs_by_pop$p1 == 0 | allele_freqs_by_pop$p1 == 1))
S <- sum(allele_freqs_by_pop$p1 >0 & allele_freqs_by_pop$p1 <1)
if(S == 0) {
warning("No segregating sites")
data.frame(pi = NaN,
S = NaN,
D = NaN,
Pval.normal = NaN,
Pval.beta = NaN)
}
n <- mean(allele_freqs_by_pop$nobs * 2 )
tmp <- 1:(n - 1)
a1 <- sum(1/tmp)
a2 <- sum(1/tmp^2)
b1 <- (n + 1)/(3 * (n - 1))
b2 <- 2 * (n^2 + n + 3)/(9 * n * (n - 1))
c1 <- b1 - 1/a1
c2 <- b2 - (n + 2)/(a1 * n) + a2/a1^2
e1 <- c1/a1
e2 <- c2/(a1^2 + a2)
#calculate D and do beta testing
D <- (pi - S/a1) / sqrt(e1 * S + e2 * S * (S - 1))
Dmin <- (2/n - 1/a1)/sqrt(e2)
Dmax <- ((n/(2*(n - 1))) - 1/a1)/sqrt(e2)
tmp1 <- 1 + Dmin * Dmax
tmp2 <- Dmax - Dmin
a <- -tmp1 * Dmax/tmp2
b <- tmp1 * Dmin/tmp2
data.frame(pi = pi,
S = S,
D = D)
}
output <- do.call("rbind", lapply(allele_freqs_by_pop,
get_tajima_D_for_one_pop))
data.frame(population = rownames(output), output, row.names = NULL)
}We are going to focus on key filters and how they impact estimates
Minor allele frequency (MAF) filtering, which explicitly removes rare alleles
No MAF allele filtering and taking all data as it comes
No MAF filtering but filtering on read depth so we are confident in our rare alleles
1. MAF filtering
We will start with our lightly cleaned data and filter this for the tajima’s calculation.
#This function is written to calculate Tajima's D with a fair amount of missing data
#so we are going to filter lightly here
gl.report.callrate(gl.clean)Starting gl.report.callrate
Processing genlight object with SNP data
Reporting Call Rate by Locus
No. of loci = 44752
No. of individuals = 36
Minimum : 0.805556
1st quartile : 0.888889
Median : 0.944444
Mean : 0.9387004
3r quartile : 1
Maximum : 1
Missing Rate Overall: 0.0613 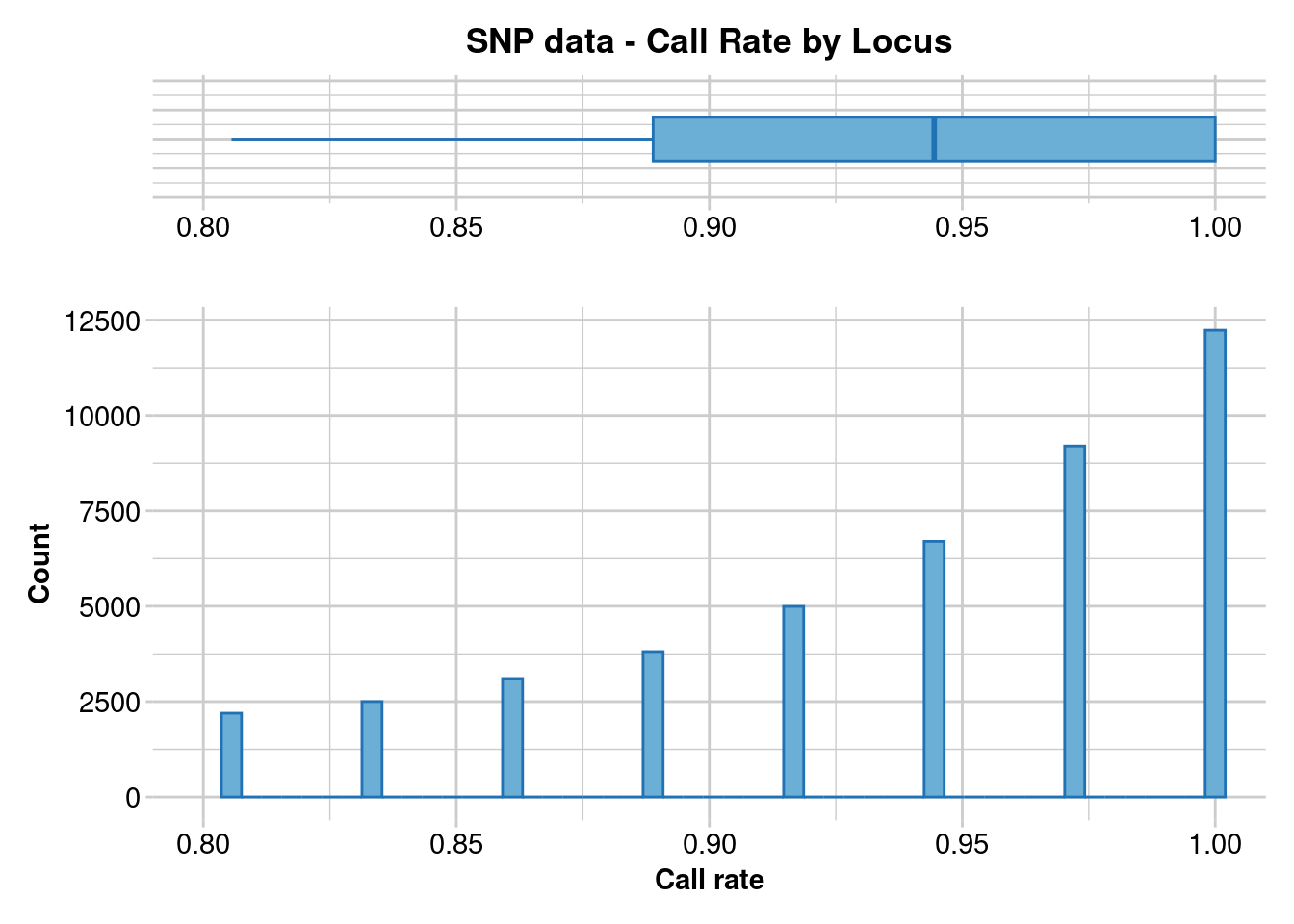
Completed: gl.report.callrate gl.1 <- gl.filter.callrate(gl.clean, method = "loc", threshold = 0.9)Starting gl.filter.callrate
Processing genlight object with SNP data
Recalculating Call Rate
Removing loci based on Call Rate, threshold = 0.9 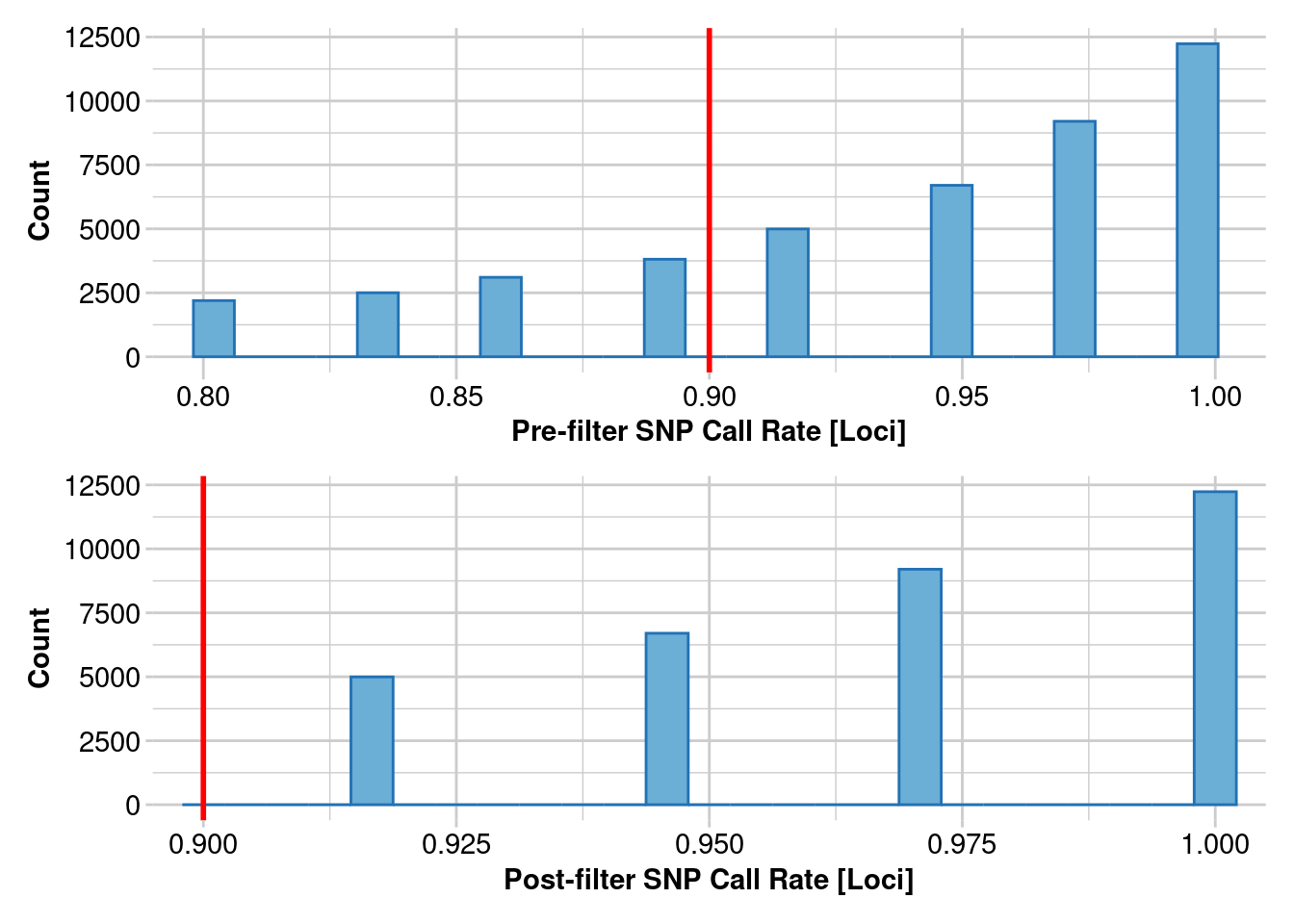
Completed: gl.filter.callrate #In this first round, we are going to actually remove singletons (our rare alleles) to see what happens
gl.report.maf(gl.1)Starting gl.report.maf
Processing genlight object with SNP data
Starting gl.report.maf
Reporting Minor Allele Frequency (MAF) by Locus for population IK
No. of loci = 14958
No. of individuals = 22
Minimum : 0.0227
1st quantile : 0.0227
Median : 0.0455
Mean : 0.09433557
3r quantile : 0.0952
Maximum : 0.5
Missing Rate Overall: 0.01
Reporting Minor Allele Frequency (MAF) by Locus for population IT
No. of loci = 24122
No. of individuals = 14
Minimum : 0.0357
1st quantile : 0.0385
Median : 0.0769
Mean : 0.1268348
3r quantile : 0.1667
Maximum : 0.5
Missing Rate Overall: 0.06
Reporting Minor Allele Frequency (MAF) by Locus OVERALL
No. of loci = 33136
No. of individuals = 36
Minimum : 0.0114
1st quantile : 0.0192
Median : 0.0384
Mean : 0.08512636
3r quantile : 0.0893
Maximum : 0.5
Missing Rate Overall: 0.03 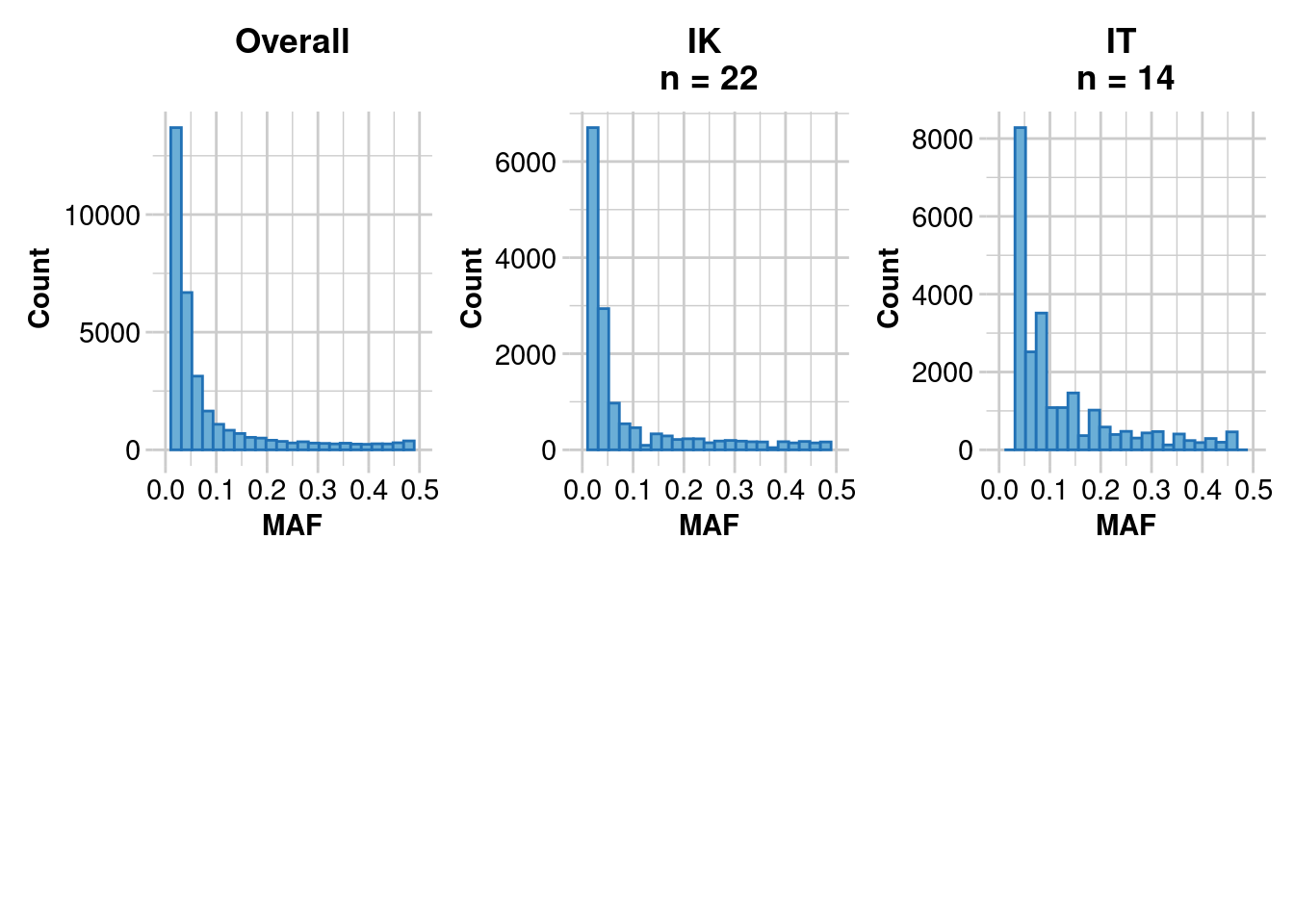
Quantile Threshold Retained Percent Filtered Percent
1 100% 0.5000 75 0.2 33061 99.8
2 95% 0.3785 1657 5.0 31479 95.0
3 90% 0.2559 3314 10.0 29822 90.0
4 85% 0.1705 4984 15.0 28152 85.0
5 80% 0.1190 6630 20.0 26506 80.0
6 75% 0.0893 8533 25.8 24603 74.2
7 70% 0.0714 10159 30.7 22977 69.3
8 65% 0.0577 11608 35.0 21528 65.0
9 60% 0.0454 13989 42.2 19147 57.8
10 55% 0.0416 15067 45.5 18069 54.5
11 50% 0.0384 16598 50.1 16538 49.9
12 45% 0.0357 18691 56.4 14445 43.6
13 40% 0.0292 19887 60.0 13249 40.0
14 35% 0.0227 22254 67.2 10882 32.8
15 30% 0.0209 23242 70.1 9894 29.9
16 25% 0.0192 25099 75.7 8037 24.3
17 20% 0.0178 28849 87.1 4287 12.9
18 15% 0.0178 28849 87.1 4287 12.9
19 10% 0.0114 32776 98.9 360 1.1
20 5% 0.0114 32776 98.9 360 1.1
21 0% 0.0114 33136 100.0 0 0.0
Completed: gl.report.maf nLoc(gl.1)[1] 33136gl.2 <- gl.filter.maf(gl.1, threshold = 0.05)Starting gl.select.colors
Warning: Number of required colors not specified, set to 9
Library: RColorBrewer
Palette: brewer.pal
Showing and returning 2 of 9 colors for library RColorBrewer : palette Blues 
Completed: gl.select.colors
Starting gl.filter.maf
Processing genlight object with SNP data
Removing loci with MAF < 0.05 over all the dataset
and recalculating FreqHoms and FreqHets
Completed: gl.filter.maf nLoc(gl.2)[1] 12823#check that the data looks fairly clean
#this starts to show some obvious banding which are the two populations
plot(gl.2)
#we are also going to remove secondary SNPs on the same fragment in this first round
gl.3 <- gl.filter.secondaries(gl.2)Starting gl.filter.secondaries
Processing genlight object with SNP data
Selecting one SNP per sequence tag at random
Completed: gl.filter.secondaries #Filter on individuals. You can usually be a bit flexible at this point.
#make note of any individuals with a low call rate. Keep them in for now
#but if they act weird in the analysis, you may want to consider removing
gl.report.callrate(gl.3, method = "ind")Starting gl.report.callrate
Processing genlight object with SNP data
Reporting Call Rate by Individual
No. of loci = 10422
No. of individuals = 36
Minimum : 0.8513721
1st quartile : 0.9509211
Median : 0.9869507
Mean : 0.9658255
3r quartile : 0.9918922
Maximum : 0.9948187
Missing Rate Overall: 0.0342
Listing 2 populations and their average CallRates
Monitor again after filtering
Population CallRate N
1 IK 0.9878 22
2 IT 0.9313 14
Listing 20 individuals with the lowest CallRates
Use this list to see which individuals will be lost on filtering by individual
Set ind.to.list parameter to see more individuals
Individual CallRate
1 Up1041_R138706 0.8513721
2 Up1186_QMJ88804 0.8919593
3 Up1096_R36195 0.9030896
4 Up0854_ABTC17229 0.9175782
5 Up0969_ABTC29129 0.9260219
6 Up1120_R36170 0.9375360
7 Up1118_NTMR36173 0.9396469
8 Up0767_ABTC12489 0.9418538
9 Up0832_ABTC93392 0.9507772
10 Up0731_ABTC99709 0.9509691
11 Up1136_NTMR36228 0.9531760
12 Up0769_ABTC12511 0.9537517
13 Up1269_WAMR164857 0.9573019
14 Up0276_NTMR35114 0.9580695
15 Up1078_R36177 0.9618116
16 Up0166_WAMR162490 0.9654577
17 Up1266_WAMR164844 0.9762042
18 Up0183_WAMR162558 0.9866628
19 Up1321_WAMR171536 0.9872385
20 WAMR162566 0.9877183
)
Completed: gl.report.callrate gl.4 <- gl.filter.callrate(gl.3, method = "ind", threshold = .9)Starting gl.filter.callrate
Processing genlight object with SNP data
Recalculating Call Rate
Removing individuals based on Call Rate, threshold = 0.9
Individuals deleted (CallRate <= 0.9 ):
Up1186_QMJ88804[IT], Up1041_R138706[IT],
Note: Locus metrics not recalculated
Note: Resultant monomorphic loci not deleted
Completed: gl.filter.callrate #Always run this after removing individuals
gl.D_withfiltering <- gl.filter.monomorphs(gl.4)Starting gl.filter.monomorphs
Processing genlight object with SNP data
Identifying monomorphic loci
Removing monomorphic loci and loci with all missing
data
Completed: gl.filter.monomorphs #calculate tajima's D with removing singletons and secondaries
gl.D_withfiltering ********************
*** DARTR OBJECT ***
********************
** 34 genotypes, 9,857 SNPs , size: 70.6 Mb
missing data: 9571 (=2.86 %) scored as NA
** Genetic data
@gen: list of 34 SNPbin
@ploidy: ploidy of each individual (range: 2-2)
** Additional data
@ind.names: 34 individual labels
@loc.names: 9857 locus labels
@loc.all: 9857 allele labels
@position: integer storing positions of the SNPs [within 69 base sequence]
@pop: population of each individual (group size range: 12-22)
@other: a list containing: loc.metrics, ind.metrics, latlon, loc.metrics.flags, verbose, history
@other$ind.metrics: id, pop, popx, lat, lon, service, plate_location
@other$loc.metrics: AlleleID, CloneID, AlleleSequence, TrimmedSequence, SNP, SnpPosition, CallRate, OneRatioRef, OneRatioSnp, FreqHomRef, FreqHomSnp, FreqHets, PICRef, PICSnp, AvgPIC, AvgCountRef, AvgCountSnp, RepAvg, clone, uid, rdepth, maf
@other$latlon[g]: coordinates for all individuals are attachedD_w_filtering <- get_tajima_D(gl.D_withfiltering)Registered S3 method overwritten by 'GGally':
method from
+.gg ggplot2Registered S3 method overwritten by 'genetics':
method from
[.haplotype pegasRegistered S3 methods overwritten by 'dartR':
method from
cbind.dartR dartR.base
rbind.dartR dartR.baseStarting ::
Starting dartR
Starting gl.percent.freq
Processing genlight object with SNP data
Starting gl.percent.freq: Calculating allele frequencies for populations
Completed: ::
Completed: dartR
Completed: gl.percent.freq D_w_filtering population pi S D
1 IK 729.9187 4809 -1.2812312
2 IT 1511.4575 6863 -0.8129841rm(gl.1, gl.2, gl.3, gl.4)2. No MAF filtering
Now lets try it where we don’t remove singletons or secondaries
#This function is written to calculate Tajima's D with a fair amount of missing data
#we are going to filter lightly here
gl.report.callrate(gl.clean)Starting gl.report.callrate
Processing genlight object with SNP data
Reporting Call Rate by Locus
No. of loci = 44752
No. of individuals = 36
Minimum : 0.805556
1st quartile : 0.888889
Median : 0.944444
Mean : 0.9387004
3r quartile : 1
Maximum : 1
Missing Rate Overall: 0.0613 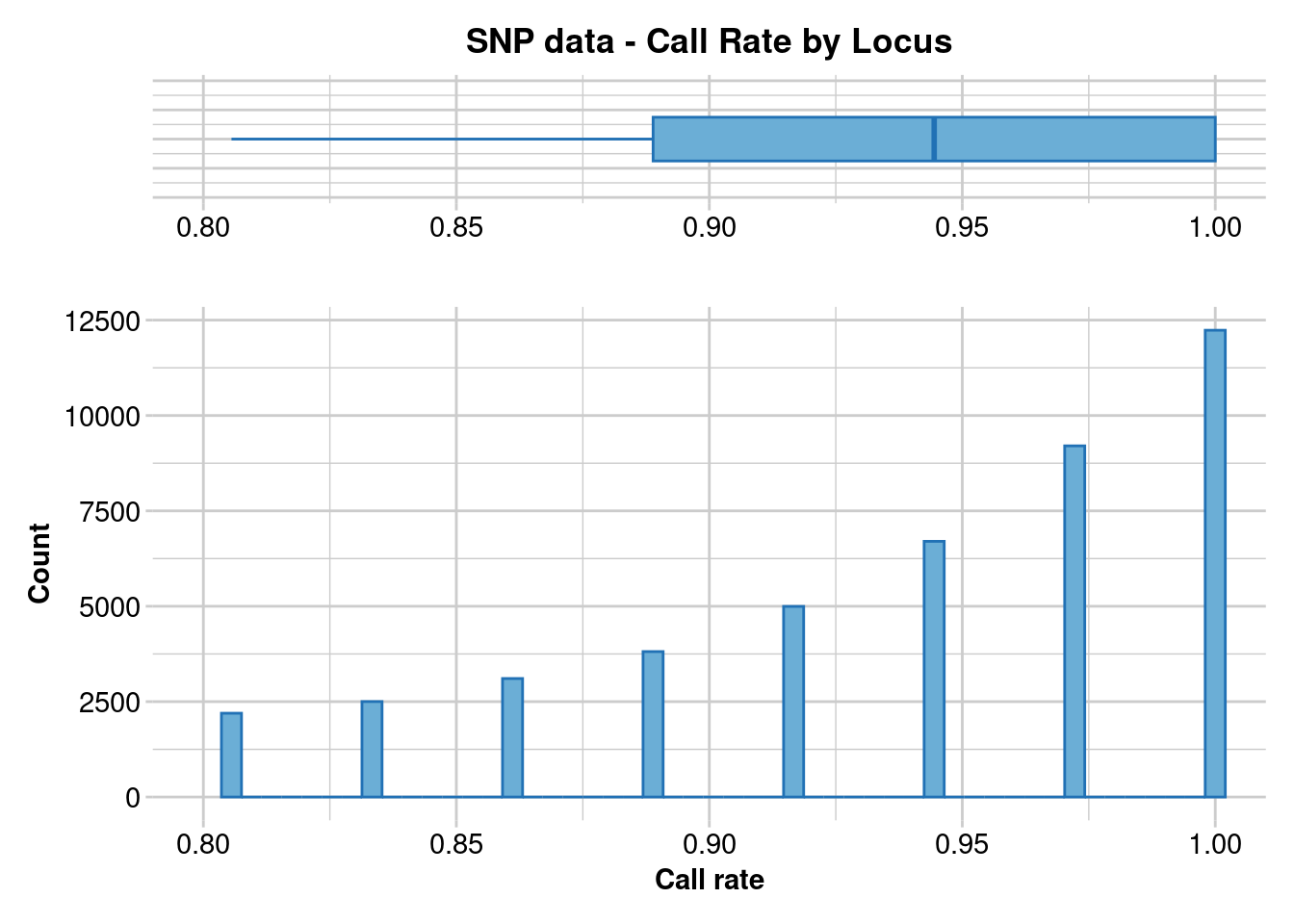
Completed: gl.report.callrate gl.1 <- gl.filter.callrate(gl.clean, method = "loc", threshold = 0.9)Starting gl.filter.callrate
Processing genlight object with SNP data
Recalculating Call Rate
Removing loci based on Call Rate, threshold = 0.9 
Completed: gl.filter.callrate #check that the data looks fairly clean
#this starts ot show some obvious population banding
plot(gl.1)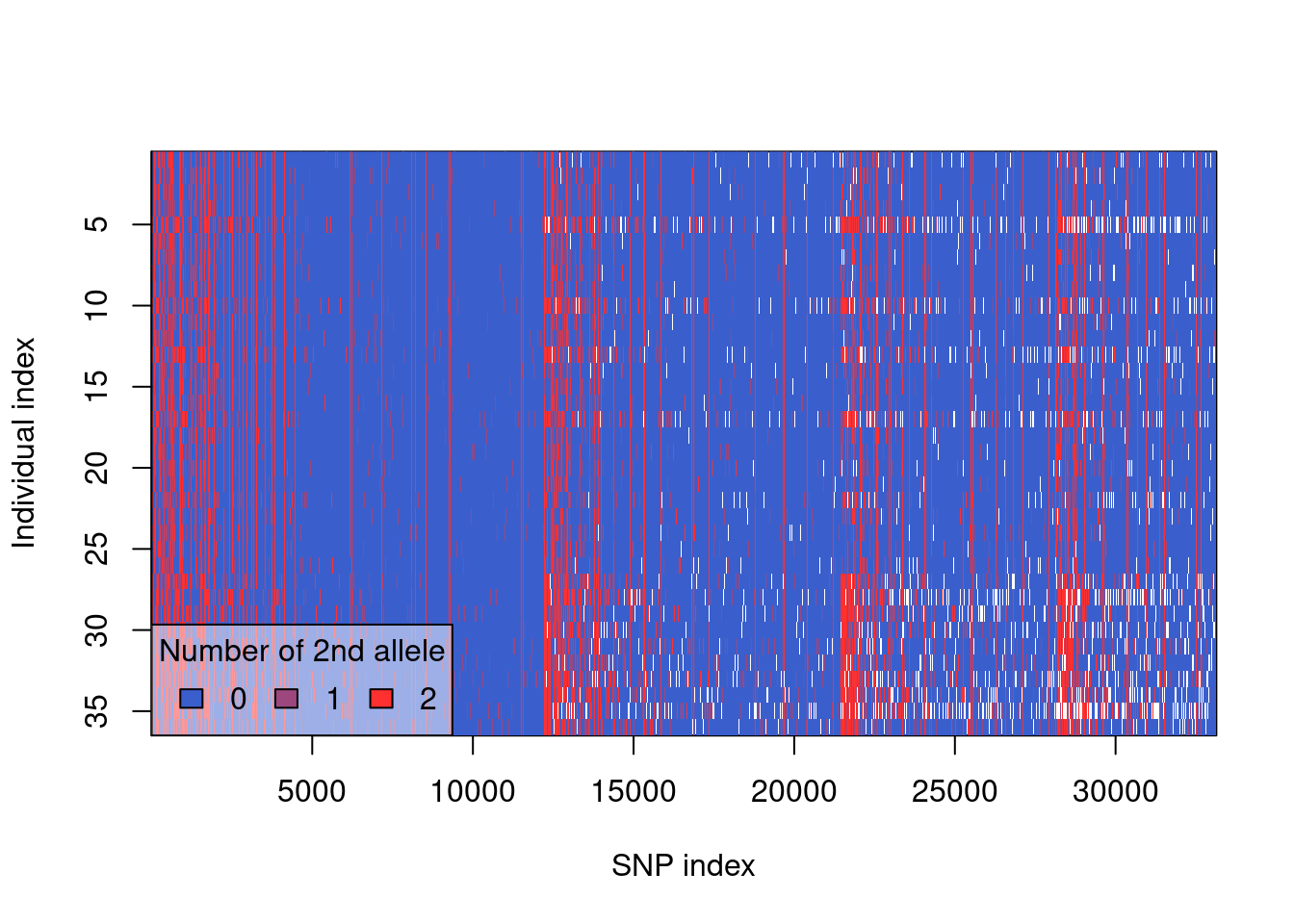
#Filter on individuals. You can usually be a bit flexible at this point.
#make note of any idnviduals with a low call rate. Keep them in for now
#but if they act weird in the analysis, you may want to consider removing
gl.report.callrate(gl.1, method = "ind")Starting gl.report.callrate
Processing genlight object with SNP data
Reporting Call Rate by Individual
No. of loci = 33136
No. of individuals = 36
Minimum : 0.8620232
1st quartile : 0.9536758
Median : 0.9886679
Mean : 0.9684834
3r quartile : 0.9923648
Maximum : 0.9943264
Missing Rate Overall: 0.0315
Listing 2 populations and their average CallRates
Monitor again after filtering
Population CallRate N
1 IK 0.9888 22
2 IT 0.9366 14
Listing 20 individuals with the lowest CallRates
Use this list to see which individuals will be lost on filtering by individual
Set ind.to.list parameter to see more individuals
Individual CallRate
1 Up1041_R138706 0.8620232
2 Up1186_QMJ88804 0.9028549
3 Up1096_R36195 0.9145039
4 Up0854_ABTC17229 0.9209319
5 Up0969_ABTC29129 0.9341803
6 Up1120_R36170 0.9400954
7 Up1118_NTMR36173 0.9462216
8 Up0767_ABTC12489 0.9478211
9 Up0731_ABTC99709 0.9527704
10 Up1136_NTMR36228 0.9539775
11 Up0832_ABTC93392 0.9551545
12 Up0276_NTMR35114 0.9586251
13 Up0769_ABTC12511 0.9594097
14 Up1269_WAMR164857 0.9621861
15 Up1078_R36177 0.9633028
16 Up0166_WAMR162490 0.9667733
17 Up1266_WAMR164844 0.9783317
18 Up1321_WAMR171536 0.9882001
19 Up0083_WAMR113846 0.9891357
20 Up0183_WAMR162558 0.9891960
)
Completed: gl.report.callrate gl.2 <- gl.filter.callrate(gl.1, method = "ind", threshold = .9)Starting gl.filter.callrate
Processing genlight object with SNP data
Recalculating Call Rate
Removing individuals based on Call Rate, threshold = 0.9
Individuals deleted (CallRate <= 0.9 ):
Up1041_R138706[IT],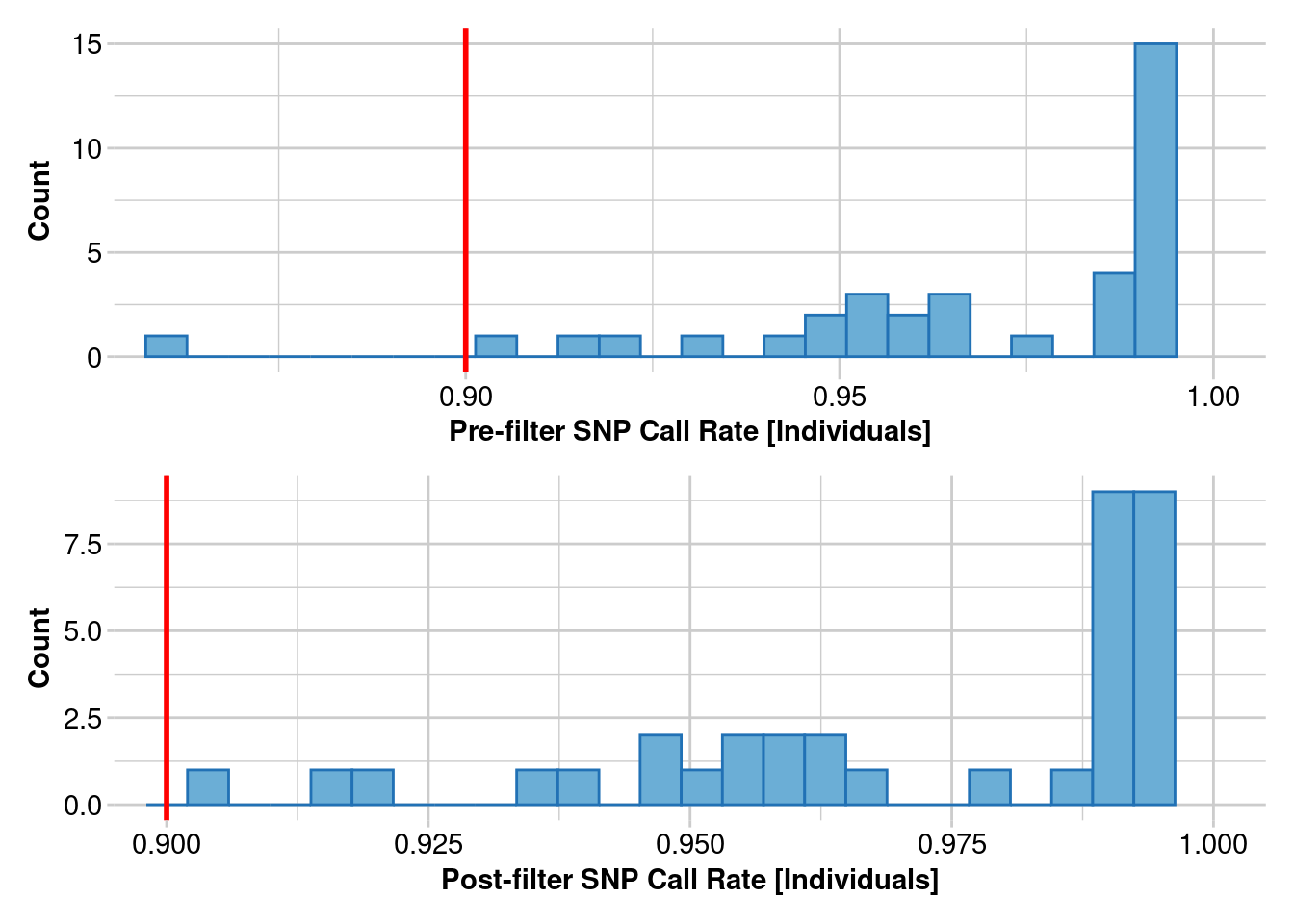
Note: Locus metrics not recalculated
Note: Resultant monomorphic loci not deleted
Completed: gl.filter.callrate #Always run this after removing individuals
gl.D_withOutfiltering <- gl.filter.monomorphs(gl.2)Starting gl.filter.monomorphs
Processing genlight object with SNP data
Identifying monomorphic loci
Removing monomorphic loci and loci with all missing
data
Completed: gl.filter.monomorphs rm(gl.1, gl.2)
D_wOUT_filtering <- get_tajima_D(gl.D_withOutfiltering)Starting ::
Starting dartR
Starting gl.percent.freq
Processing genlight object with SNP data
Starting gl.percent.freq: Calculating allele frequencies for populations
Completed: ::
Completed: dartR
Completed: gl.percent.freq 3. No MAF filtering but filtering on Read depth
Now lets try it where we don’t remove singletons or secondaries AND we filter for read depth to remove singletons where we are not confident about their base calls
#This function is written to calculate Tajima's D with a fair amount of missing data
# we are going to filter lightly here
gl.report.callrate(gl.clean)Starting gl.report.callrate
Processing genlight object with SNP data
Reporting Call Rate by Locus
No. of loci = 44752
No. of individuals = 36
Minimum : 0.805556
1st quartile : 0.888889
Median : 0.944444
Mean : 0.9387004
3r quartile : 1
Maximum : 1
Missing Rate Overall: 0.0613 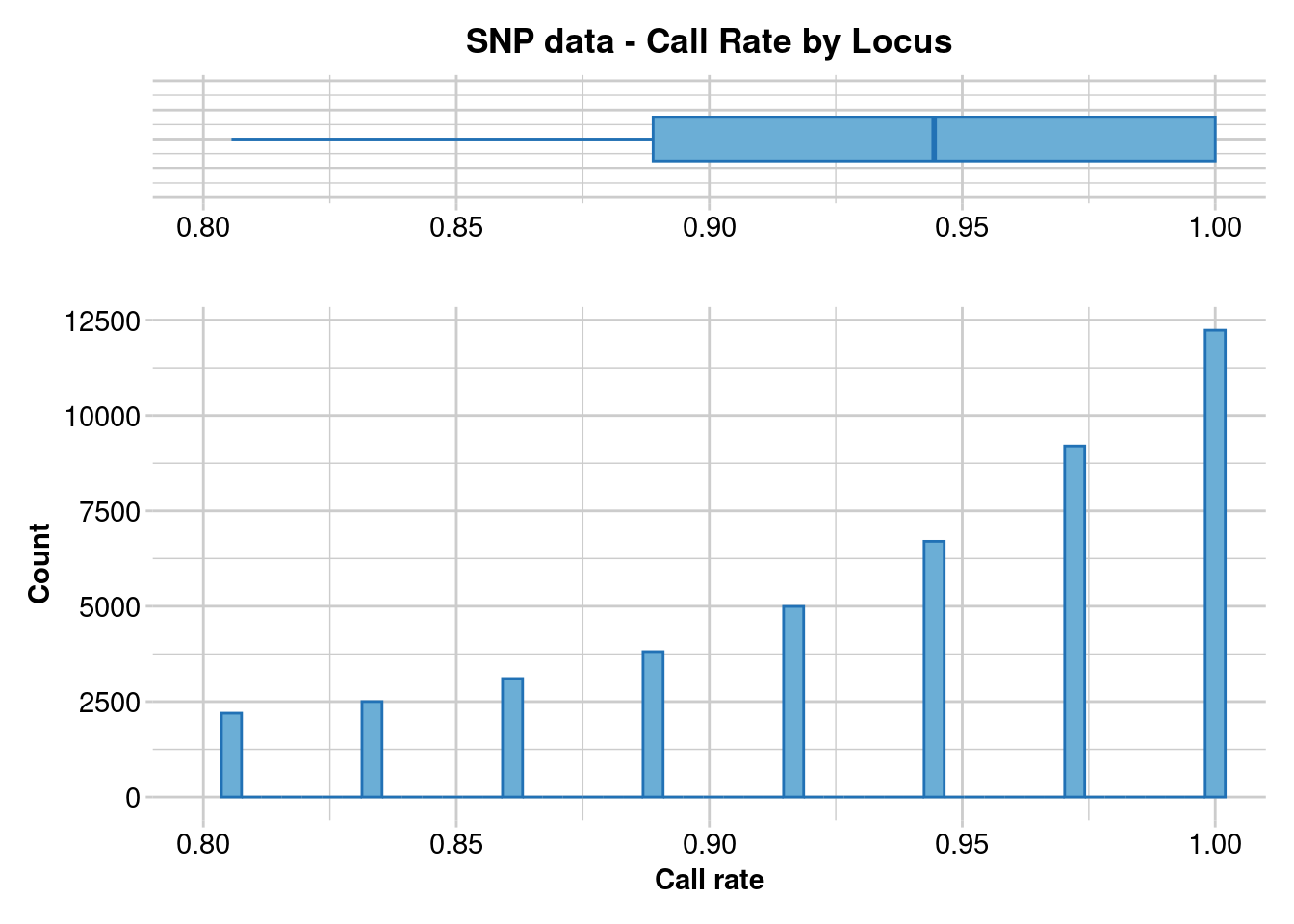
Completed: gl.report.callrate gl.1 <- gl.filter.callrate(gl.clean, method = "loc", threshold = 0.9)Starting gl.filter.callrate
Processing genlight object with SNP data
Recalculating Call Rate
Removing loci based on Call Rate, threshold = 0.9 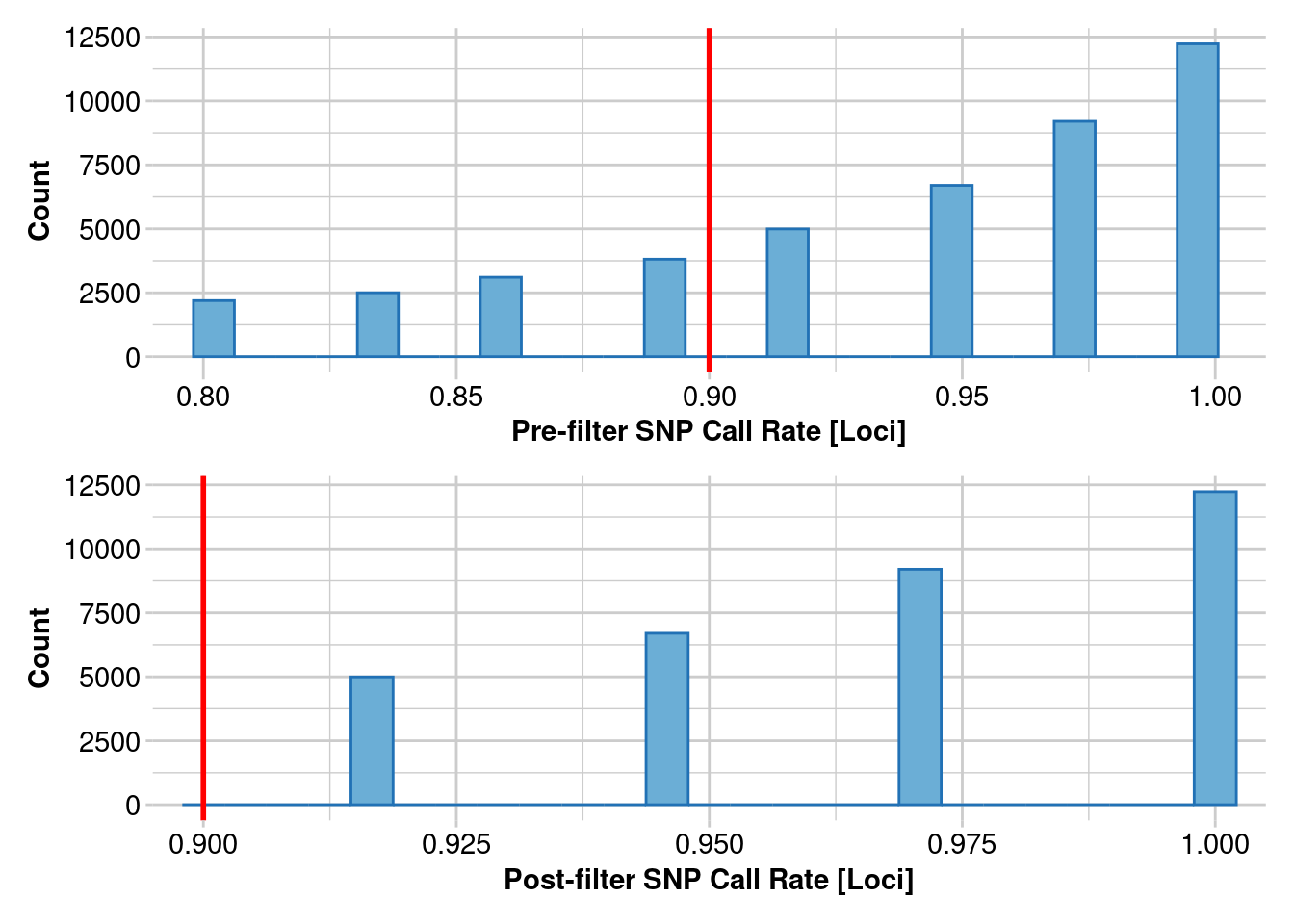
Completed: gl.filter.callrate #check that the data looks fairly clean
#this starts ot show some obvious population banding
plot(gl.1)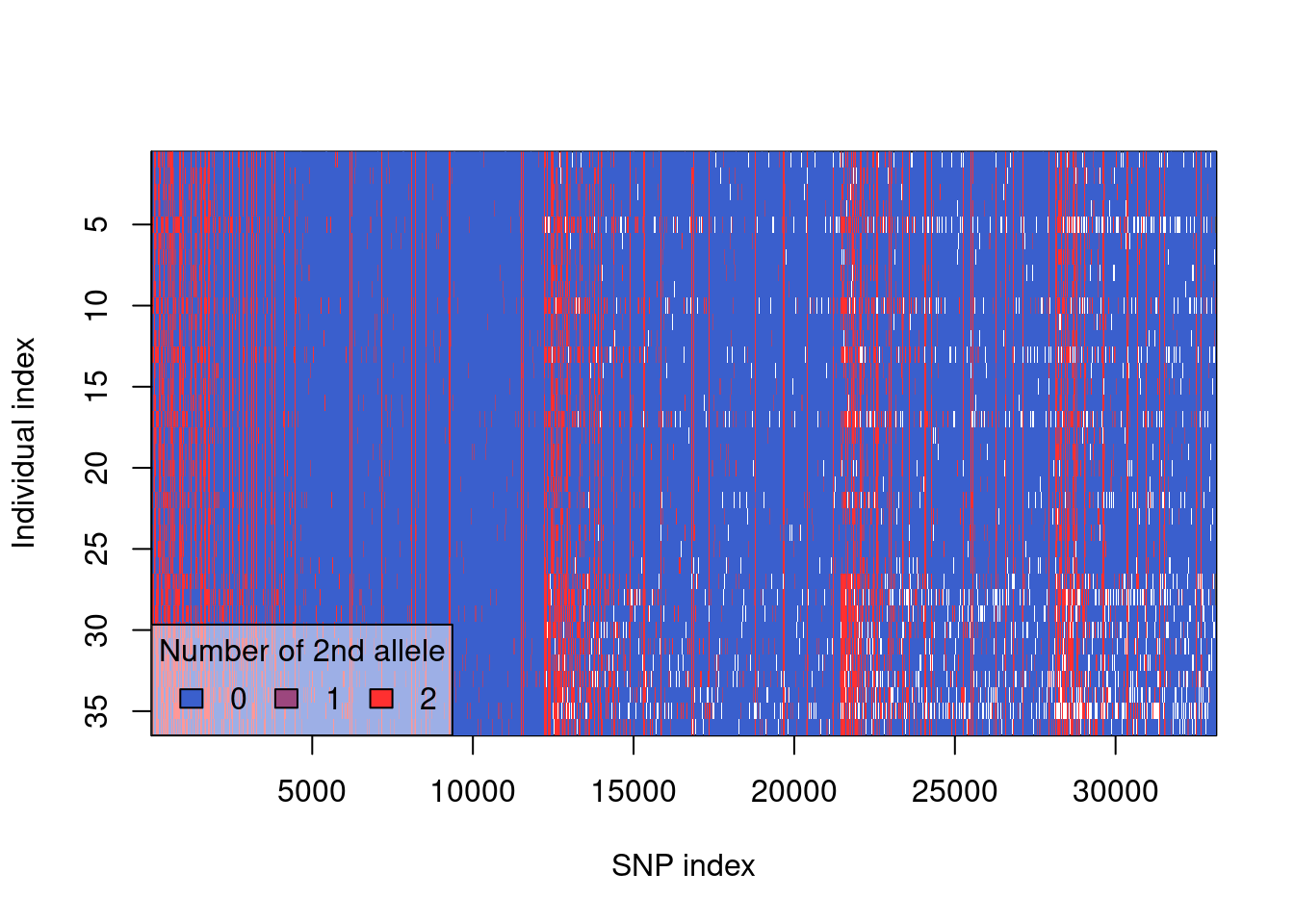
#filter to loci with a lower read depth, so that we are really confident
#that our base calls are correct
gl.report.rdepth(gl.1)Starting gl.report.rdepth
Processing genlight object with SNP data
Reporting Read Depth by Locus
No. of loci = 33136
No. of individuals = 36
Minimum : 8
1st quartile : 9.3
Median : 10.7
Mean : 10.94332
3r quartile : 12.4
Maximum : 17
Missing Rate Overall: 0.03 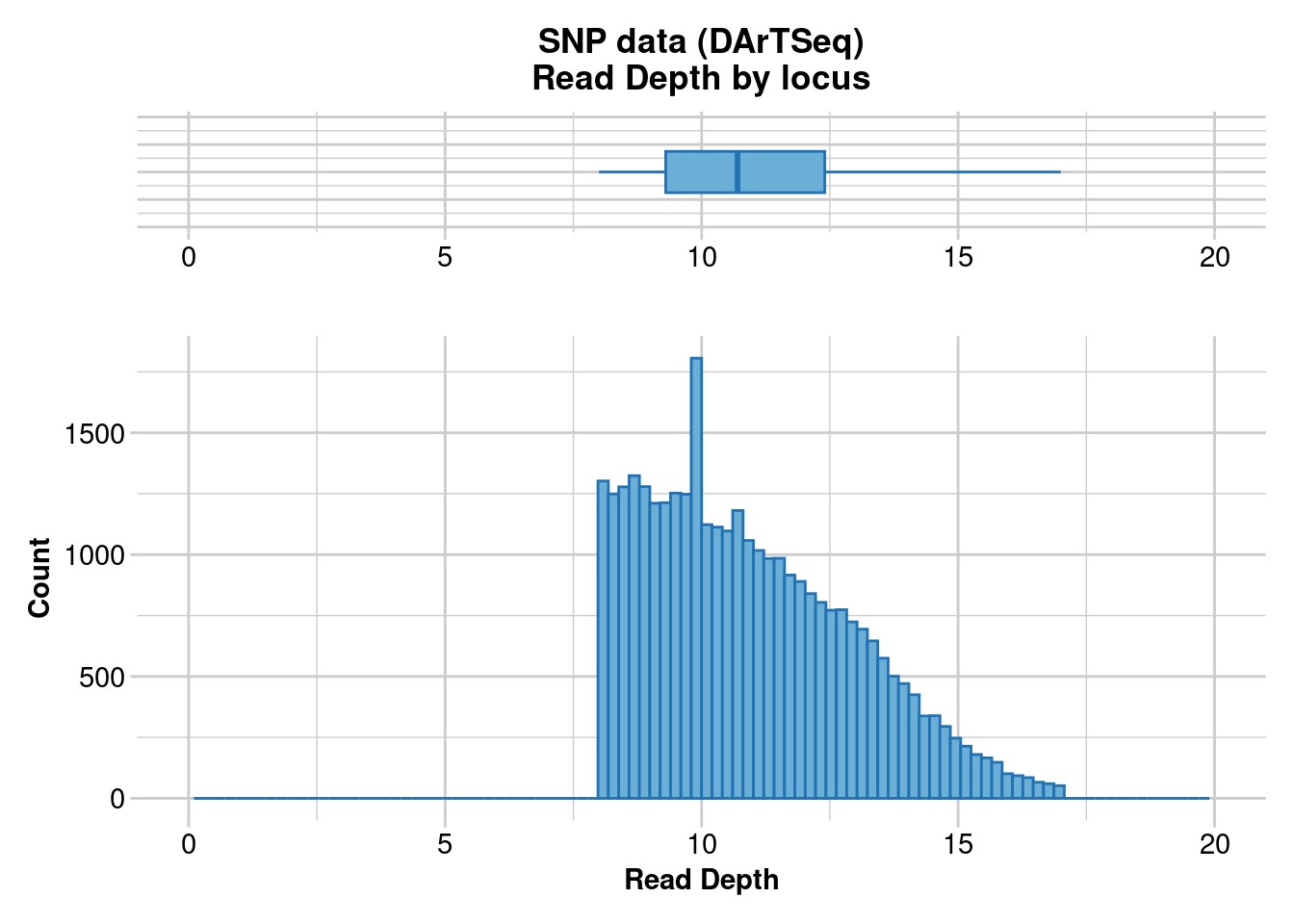
Quantile Threshold Retained Percent Filtered Percent
1 100% 17.0 27 0.1 33109 99.9
2 95% 14.7 1707 5.2 31429 94.8
3 90% 13.8 3536 10.7 29600 89.3
4 85% 13.3 5002 15.1 28134 84.9
5 80% 12.8 6811 20.6 26325 79.4
6 75% 12.4 8369 25.3 24767 74.7
7 70% 12.0 10014 30.2 23122 69.8
8 65% 11.6 11913 36.0 21223 64.0
9 60% 11.3 13385 40.4 19751 59.6
10 55% 11.0 14934 45.1 18202 54.9
11 50% 10.7 16641 50.2 16495 49.8
12 45% 10.4 18337 55.3 14799 44.7
13 40% 10.1 19974 60.3 13162 39.7
14 35% 9.8 21780 65.7 11356 34.3
15 30% 9.5 23645 71.4 9491 28.6
16 25% 9.3 24859 75.0 8277 25.0
17 20% 9.0 26704 80.6 6432 19.4
18 15% 8.7 28644 86.4 4492 13.6
19 10% 8.5 29974 90.5 3162 9.5
20 5% 8.2 31834 96.1 1302 3.9
21 0% 8.0 33136 100.0 0 0.0
Completed: gl.report.rdepth gl.2 <- gl.filter.rdepth(gl.1, lower = 12, upper = 17)Starting gl.filter.rdepth
Processing genlight object with SNP data
Removing loci with rdepth <= 12 and >= 17 
Completed: gl.filter.rdepth #Filter on individuals. You can usually be a bit flexible at this point.
#make note of any idnviduals with a low call rate. Keep them in for now
#but if they act weird in the analysis, you may want to consider removing
gl.report.callrate(gl.2, method = "ind")Starting gl.report.callrate
Processing genlight object with SNP data
Reporting Call Rate by Individual
No. of loci = 10014
No. of individuals = 36
Minimum : 0.8722788
1st quartile : 0.9646245
Median : 0.9926103
Mean : 0.9749822
3r quartile : 0.9955063
Maximum : 0.9969043
Missing Rate Overall: 0.025
Listing 2 populations and their average CallRates
Monitor again after filtering
Population CallRate N
1 IK 0.9926 22
2 IT 0.9473 14
Listing 20 individuals with the lowest CallRates
Use this list to see which individuals will be lost on filtering by individual
Set ind.to.list parameter to see more individuals
Individual CallRate
1 Up1041_R138706 0.8722788
2 Up1186_QMJ88804 0.9191132
3 Up1096_R36195 0.9281007
4 Up0854_ABTC17229 0.9320951
5 Up0969_ABTC29129 0.9398842
6 Up1120_R36170 0.9531656
7 Up1118_NTMR36173 0.9573597
8 Up0767_ABTC12489 0.9620531
9 Up0832_ABTC93392 0.9636509
10 Up1078_R36177 0.9649491
11 Up0731_ABTC99709 0.9657480
12 Up0769_ABTC12511 0.9662473
13 Up1136_NTMR36228 0.9678450
14 Up0276_NTMR35114 0.9690433
15 Up1269_WAMR164857 0.9721390
16 Up0166_WAMR162490 0.9740363
17 Up1266_WAMR164844 0.9870182
18 Up1321_WAMR171536 0.9924106
19 WAMR162566 0.9928101
20 Up0183_WAMR162558 0.9928101
)
Completed: gl.report.callrate gl.3 <- gl.filter.callrate(gl.2, method = "ind", threshold = .9)Starting gl.filter.callrate
Processing genlight object with SNP data
Recalculating Call Rate
Removing individuals based on Call Rate, threshold = 0.9
Individuals deleted (CallRate <= 0.9 ):
Up1041_R138706[IT],
Note: Locus metrics not recalculated
Note: Resultant monomorphic loci not deleted
Completed: gl.filter.callrate #Always run this after removing individuals
gl.D_withOutfilteringRDepth <- gl.filter.monomorphs(gl.3)Starting gl.filter.monomorphs
Processing genlight object with SNP data
Identifying monomorphic loci
Removing monomorphic loci and loci with all missing
data
Completed: gl.filter.monomorphs rm(gl.1, gl.2, gl.3)
#calculate tajima's D with removing singletons and secondaries
D_wOUT_filtering_Rdepth <- get_tajima_D(gl.D_withOutfilteringRDepth)Starting ::
Starting dartR
Starting gl.percent.freq
Processing genlight object with SNP data
Starting gl.percent.freq: Calculating allele frequencies for populations
Completed: ::
Completed: dartR
Completed: gl.percent.freq Results
lets look at all three
D_w_filtering population pi S D
1 IK 729.9187 4809 -1.2812312
2 IT 1511.4575 6863 -0.8129841D_wOUT_filtering population pi S D
1 IK 2191.305 14958 -1.3671682
2 IT 4788.202 22787 -0.8746524D_wOUT_filtering_Rdepth population pi S D
1 IK 674.2647 4643 -1.3871722
2 IT 1494.1162 6981 -0.8153518For our Kimberley population, removing minor alleles give the actual wrong answer and suggests the population is contracting. This would have contradicted the rest of the findings of the paper, and been an artifact of the filtering process.
This shows how understanding the population genetic theory for a metric is crucial to useful analyses.
Further Study
Another teaching app in R, covering only Shannon for genes and its change over time, produced by Computer Science and Engineering third-year students: https://evolutionaryecology.shinyapps.io/learningEE
Readings
O’Reilly et al. (2020)
Sherwin (2022)
Sherwin et al. (2017)
Sherwin et al. (2021)
Jaya et al. (2022)